| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Ceftriaxone is only given as an injection, either intramuscularly or intravenously. Ceftriaxone is less than 1% bioavailable if given orally. Ceftriaxone is primarily eliminated in the urine (33-67%). The remainder is eliminated through secretion in the bile and removed from the body via the feces. The apparent volume of distribution of an intravenous or intramuscular dose in healthy patients is 5.78 to 13.5 L. The volume of distribution of an intravenous or intramuscular dose in septic patients is 6.48 to 35.2 L. Ceftriaxone has good enough CSF penetration to be used as an effective treatment of bacterial meningitis. The plasma clearance of ceftriaxone in healthy adults receiving a 0.15-3g dose is 0.58 to 1.45 L/hour. The renal clearance of ceftriaxone is 0.32 to 0.73 L/hour. In intensive care unit patients, ceftriaxone's total drug clearance was 0.96L/h (0.55-1.28 L/h), and unbound drug clearance was 1.91 L/h (1.46-6.20 L/h). Metabolism / Metabolites Metabolism of ceftriaxone is negligible. Ceftriaxone is eliminated unchanged in the urine by glomerular filtration (60%) and bile (40%) (A633). Route of Elimination: Thirty-three percent to 67% of a ceftriaxone dose was excreted in the urine as unchanged drug and the remainder was secreted in the bile and ultimately found in the feces as microbiologically inactive compounds. Half Life: 5.8-8.7 hours Biological Half-Life The elimination half-life of ceftriaxone is 5.8-8.7 hours. The half-life of ceftriaxone in the middle ear fluid has been estimated to be 25 hours. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Ceftriaxone works by inhibiting the mucopeptide synthesis in the bacterial cell wall. The beta-lactam moiety of Ceftriaxone binds to carboxypeptidases, endopeptidases, and transpeptidases in the bacterial cytoplasmic membrane. These enzymes are involved in cell-wall synthesis and cell division. By binding to these enzymes, Ceftriaxone results in the formation of of defective cell walls and cell death. Hepatotoxicity Parenteral administration of ceftriaxone has been associated with development of biliary sludge in 3% to 46% of patients. The incidence may be higher in children than adults and is associated with higher doses and longer courses of treatment and possibly with fasting or dehydration. The syndrome is referred to as “pseudolithiasis” as the sludge and stones consist largely of ceftriaxone and they resolve spontaneously when the drug is stopped, indicating that surgery can be avoided. Most cases occur with minimal or no symptoms. Frank symptoms of cholecystitis are reported in up to 5% of patients who develop pseudo-lithiasis. Typically, serum enzymes and bilirubin levels remain normal even with biliary colic, but in rare instances there is cholestatic jaundice or gallstone pancreatitis that can be severe and require surgical intervention. Sludge and symptoms of gallbladder disease can arise within a few days of starting therapy, but typically resolve rapidly once ceftriaxone is stopped, although sludge and gallstones may be detectable by ultrasound for several months. Ceftriaxone can also lead to an immunoallergic form of cholestatic hepatitis similar to what has been described with other cephalosporins. This reaction is idiosyncratic and is very rare. Symptoms of abdominal pain, nausea, pruritis and jaundice arise within 1 to 4 weeks of initiation of therapy and may worsen for 1 to 2 weeks after stopping the antibiotic. A cholestatic pattern of serum enzyme elevations and immunoallergic features of fever, rash and eosinophilia are common. The injury is usually mild and self-limited. Likelihood score: B (ceftriaxone is a very likely cause of clinically apparent liver injury and can also lead to biliary sludge and “pseudolithiasis” caused by crystallization of ceftriaxone in bile present in the gallbladder or biliary tree). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited information indicates that ceftriaxone produce low levels in milk, which are not expected to cause adverse effects in breastfed infants. Occasionally disruption of the infant's gastrointestinal flora, resulting in diarrhea or thrush have been reported with cephalosporins, but these effects have not been adequately evaluated. Ceftriaxone is acceptable in nursing mothers. ◉ Effects in Breastfed Infants A mother who was exclusively nursing her 52-day-old infant developed a soft-tissue infection. She was treated with intravenous teicoplanin 400 mg every 12 hours for 3 doses, then 400 mg daily for 5 days total, intravenous ceftriaxone 1 gram daily, topical mupirocin cream twice daily. A careful follow-up indicated that her infant had no adverse effects. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Ceftriaxone is 95% protein bound. Toxicity Data LD50: >10 000 mg/kg (Oral, Rat) |
| 参考文献 |
:J Biol Chem, 2008. 283(19): p. 13116-23.
|
| 其他信息 |
Pharmacodynamics
Ceftriaxone is a cephalosporin/cephamycin beta-lactam antibiotic used in the treatment of bacterial infections caused by susceptible, usually gram-positive, organisms. Ceftriaxone has in vitro activity against gram-positive aerobic, gram-negative aerobic, and anaerobic bacteria. The bactericidal activity of ceftriaxone results from the inhibition of cell wall synthesis and is mediated through ceftriaxone binding to penicillin-binding proteins (PBPs). Ceftriaxone is stable against hydrolysis by a variety of beta-lactamases, including penicillinases, and cephalosporinases and extended-spectrum beta-lactamases. However, resistance to ceftriaxone usually occurs through beta-lactamase hydrolysis, altered PBPs, or reduced bacterial cell permeability. Ceftriaxone should not be mixed with or giving in the same IV line as diluents/products containing calcium as they may cause ceftriaxone to precipitate. Ceftriaxone use may also cause biliary sludge or gallbladder pseudolithiasis. |
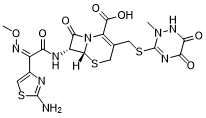
| 分子式 |
C18H18N8O7S3
|
|---|---|
| 分子量 |
554.579918384552
|
| 精确质量 |
554.046
|
| CAS号 |
73384-59-5
|
| 相关CAS号 |
Ceftriaxone sodium hydrate;104376-79-6;Ceftriaxone sodium salt;74578-69-1;Ceftriaxone-d3 disodium;1132650-38-4
|
| PubChem CID |
5479530
|
| 外观&性状 |
Off-white to yellow solid powder
|
| 密度 |
2.0±0.1 g/cm3
|
| 熔点 |
155 °C
|
| 折射率 |
1.889
|
| LogP |
-0.77
|
| tPSA |
293.8
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
13
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
36
|
| 分子复杂度/Complexity |
1110
|
| 定义原子立体中心数目 |
2
|
| SMILES |
CN1C(=NC(=O)C(=O)N1)SCC2=C(N3[C@@H]([C@@H](C3=O)NC(=O)/C(=N\OC)/C4=CSC(=N4)N)SC2)C(=O)O
|
| InChi Key |
VAAUVRVFOQPIGI-SPQHTLEESA-N
|
| InChi Code |
InChI=1S/C18H18N8O7S3/c1-25-18(22-12(28)13(29)23-25)36-4-6-3-34-15-9(14(30)26(15)10(6)16(31)32)21-11(27)8(24-33-2)7-5-35-17(19)20-7/h5,9,15H,3-4H2,1-2H3,(H2,19,20)(H,21,27)(H,23,29)(H,31,32)/b24-8-/t9-,15-/m1/s1
|
| 化学名 |
(6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-3-[(2-methyl-5,6-dioxo-1H-1,2,4-triazin-3-yl)sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~31.25 mg/mL (~56.35 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.51 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.51 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.51 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8032 mL | 9.0158 mL | 18.0317 mL | |
| 5 mM | 0.3606 mL | 1.8032 mL | 3.6063 mL | |
| 10 mM | 0.1803 mL | 0.9016 mL | 1.8032 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Efficacy of Immunization With 4C-MenB in Preventing Experimental Urethral Infection With Neisseria Gonorrhoeae
CTID: NCT05294588
Phase: Phase 2 Status: Recruiting
Date: 2024-09-19