| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
MNK1 (IC50 = 2.2 μM)
|
|---|---|
| 体外研究 (In Vitro) |
CGP57380 在细胞测定中抑制 eIF4E 磷酸化,IC50 约为 3 μM。使用 CGP57380 去磷酸化 eIF4E 会导致 293 细胞表达更多帽依赖性报告基因。 [1] CGP57380 以剂量依赖性方式抑制蛋白质合成、VSMC 肥大和 Ang II 刺激的 eIF4E 磷酸化。 [2]在小鼠胚胎成纤维细胞 (MEF) 中,CGP57380 使野生型细胞更容易受到血清撤除引起的细胞凋亡的影响。 [3] CGP57380 阻止 BC 祖细胞重复复制。[4]
|
| 体内研究 (In Vivo) |
CGP57380 (40 mg/kg/d ip) 可有效消除 BC CML 细胞作为 LSC 和连续移植免疫缺陷小鼠的功能。 [4]
在这里,我们如前所述,对BC样本中的GMP进行FACS分选,并将其注射到8至12周龄的雌性NSG小鼠体内(图S8A)。移植后6周,用DMSO、CGP57380或达沙替尼治疗移植小鼠3周(每个治疗组n=5只小鼠)。在治疗期结束时,杀死所有小鼠,并使用免疫磁珠从造血组织中获得人类细胞。我们发现,每个治疗组的外周血或骨髓中CD45+人细胞的百分比没有差异(图6A)。然而,我们观察到达沙替尼和CGP57380对承诺的BC祖细胞具有特异性活性,因为与对照组相比,它们显著减少了BM中检测到的集落形成单位的数量(分别为P≤0.05和P≤0.005),尽管CGP57370的效果更大(图6B)。然后将从原代小鼠获得的人类细胞移植到次级受体中,并在16周内通过流式细胞术监测植入情况。到4周时,我们能够检测到三个治疗组中所有动物的移植物(图6C)。在DMSO或达沙替尼治疗的动物中,在整个16周的实验时间框架内,移植率保持在80%(即五只动物中的四只),但相比之下,CGP57380治疗的小鼠均无法保持长期移植(图6C)。16周时,对小鼠实施安乐死,并检查BM中是否存在BCR-ABL1。在用DMSO或达沙替尼治疗的每只动物中都检测到BCR-ABL1转录本(即每个治疗组五只动物中有四只),而在CGP57380治疗组的四只动物中只有一只检测到非常微弱的条带(图6D)。使用来自不同个体的CD34+BC细胞重复该实验,得到了类似的结果(图S8 B-J)。综上所述,我们的研究结果表明,体内MNK抑制可以有效地消除BC CML细胞连续移植免疫缺陷小鼠的能力,并作为LSCs发挥作用[4]。 |
| 酶活实验 |
CGP 57380 是 MNK1 的有效抑制剂,IC50 值为 2.2 μM。
MNK1和PRAK通过与活化的p38预孵育而磷酸化,p38是通过与重组MKK6b孵育而产生的(E)。制备重组激酶和eIF4E,并如前所述进行体外激酶反应。使用Oligotex Direct mRNA试剂盒从293细胞中纯化Poly(A)+mRNA。对于体外翻译,根据制造商的说明,在每毫升3或10μg激酶、[35S]蛋氨酸(0.6 mCi/ml)、1.5 mM乙酸镁、75 mM KCl、2 mM DTT和100μM ATP的存在下,用每毫升10μg mRNA对兔网织红细胞裂解物(Promega)进行编程。注意确保所有测定中的缓冲条件相同。翻译反应在30°C下孵育90分钟,并测量掺入TCA可沉淀材料的放射性。[1] 重组p38异构体在以下条件下被Mkk6(E)激活:p38(100 ng/mL)、Mkk6。Mnk1活性的典型测定反应包含激酶缓冲液中的Mnk1(2ng/mL)、HA-eIF4E(10ng/mL)、ATP(300mM)。通过加入活化的p38(0.03-3ng/mL)开始反应,并在30°C下30分钟后通过加入SDS负载缓冲液停止反应。在相同的测定条件下鉴定Mnk1抑制剂,除了在暴露于底物和抑制剂之前使用活性p38a预激活Mnk1[1]。 |
| 细胞实验 |
重组 p38 同种型在以下条件下被 Mkk6(E) 激活:p38 (100 ng/mL)、Mkk6(E) (30 ng/mL)、ATP (100 mM) 在激酶缓冲液(25 mM Hepes、25 mM b-甘油磷酸盐、0.1 mM 原钒酸钠、25 mM MgCl2、2.5 mM DTT,pH 7.4)并在 30°C 下孵育 30 分钟。 Mnk1 (2 ng/mL)、HA-eIF4E (10 ng/mL) 和 ATP (300 mM) 是 Mnk1 活性的典型检测反应的主要成分。添加活化的 p38 (0.03–3 ng/mL) 启动反应,30 分钟后在 30°C 下添加 SDS 上样缓冲液终止反应。使用相同的测定条件来鉴定 Mnk1 抑制剂,但在暴露于底物和抑制剂之前,首先使用活性 p38a 预激活 Mnk1。
|
| 动物实验 |
CD34+ cells (5×105) or GMPs (1×105) are resuspended in 25 μL 1% FBS/PBS solution and injected into the right femur of 8- to 10-wk-old sublethally irradiated (200 cGy) female mice (n=5 mice per group). For each experiment, 1% FBS/PBS solution-injected mice serve as the sham control. Using flow cytometry, mice are examined every 4 weeks after the transplant to see if human cells have grafted. Engrafted mice are treated for 3 weeks with CGP57380 (40 mg/kg/d) intraperitoneally, dasatinib (5 mg/kg/d) by gavage, or vehicle alone (n = 5 mice per group) after 6 weeks following transplantation. After the course of treatment is complete, mice are put down, and CD45+ cells are extracted from the BM and spleen using anti-human CD45-specific immunomagnetic microbeads. In the colony forming cell (CFC) assay, an aliquot of 1×105 human CD45+ cells is seeded into methylcellulose, and colonies are counted after 2 weeks. The remaining human cells from each primary transplant recipient are then all intrafemorally injected into secondary recipients, and human engraftment is monitored every two weeks starting at four weeks. All mice are put to death after 16 weeks. RT-PCR is used to find BCR-ABL1 transcripts, and flow cytometry is used to evaluate engraftment in BM and blood.
|
| 参考文献 |
|
| 其他信息 |
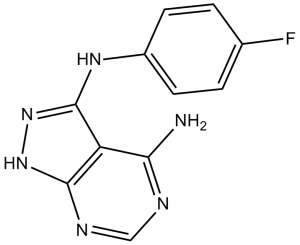
N3-(4-fluorophenyl)-2H-pyrazolo[3,4-d]pyrimidine-3,4-diamine is a pyrazolopyrimidine.
Eukaryotic initiation factor 4E (eIF4E) is a key component of the translational machinery and an important modulator of cell growth and proliferation. The activity of eIF4E is thought to be regulated by interaction with inhibitory binding proteins (4E-BPs) and phosphorylation by mitogen-activated protein (MAP) kinase-interacting kinase (MNK) on Ser209 in response to mitogens and cellular stress. Here we demonstrate that phosphorylation of eIF4E via MNK1 is mediated via the activation of either the Erk or p38 pathway. We further show that expression of active mutants of MNK1 and MNK2 in 293 cells diminishes cap-dependent translation relative to cap-independent translation in a transient reporter assay. The same effect on cap-dependent translation was observed when MNK1 was activated by the Erk or p38 pathway. In line with these findings, addition of recombinant active MNK1 to rabbit reticulocyte lysate resulted in a reduced protein synthesis in vitro, and overexpression of MNK2 caused a decreased rate of protein synthesis in 293 cells. By using CGP57380, a novel low-molecular-weight kinase inhibitor of MNK1, we demonstrate that eIF4E phosphorylation is not crucial to the formation of the initiation complex, mitogen-stimulated increase in cap-dependent translation, and cell proliferation. Our results imply that activation of MNK by MAP kinase pathways does not constitute a positive regulatory mechanism to cap-dependent translation. Instead, we propose that the kinase activity of MNKs, eventually through phosphorylation of eIF4E, may serve to limit cap-dependent translation under physiological conditions. [1] Angiotensin II (Ang II) stimulates protein synthesis in vascular smooth muscle cells (VSMCs), possibly secondary to regulatory changes at the initiation of mRNA translation. Mitogen-activated protein (MAP) kinase signal-integrating kinase-1 (Mnk1), a substrate of ERK and p38 MAP kinase, phosphorylates eukaryotic initiation factor 4E (eIF4E), an important factor in translation. The goal of the present study was to investigate the role of Mnk1 in Ang II-induced protein synthesis and to characterize the molecular mechanisms by which Mnk1 and eIF4E is activated in rat VSMCs. Ang II treatment resulted in increased Mnk1 activity and eIF4E phosphorylation. Expression of a dominant-negative Mnk1 mutant abolished Ang II-induced eIF4E phosphorylation. PD98059 or introduction of kinase-inactive MEK1/MKK1, but not SB202190 or kinase-inactive p38 MAP kinase, inhibited Ang II-induced Mnk1 activation and eIF4E phosphorylation, suggesting that ERK, but not p38 MAP kinase, is required for Ang II-induced Mnk1-eIF4E activation. Further, dominant-negative constructs for Ras, but not for Rho, Rac, or Cdc42, abolished Ang II-induced Mnk1 activation. Finally, treatment of VSMCs with CGP57380, a novel specific kinase inhibitor of Mnk1, resulted in dose-dependent decreases in Ang II-stimulated phosphorylation of eIF4E, protein synthesis, and VSMC hypertrophy. In summary, these data demonstrated that (1) Ang II-induced Mnk1 activation is mediated by the Ras-ERK cascade in VSMCs, and (2) Mnk1 is involved in Ang II-mediated protein synthesis and hypertrophy, presumably through the activation of translation-initiation. The Mnk1-eIF4E pathway may provide new insights into molecular mechanisms involved in vascular hypertrophy and other Ang II-mediated pathological states. [2] Map kinase-interacting protein kinases 1 and 2 (MNK1, MNK2) function downstream of p38 and ERK MAP kinases, but there are large gaps in our knowledge of how MNKs are regulated and function. Mice deleted of both genes are apparently normal, suggesting that MNKs function in adaptive pathways during stress. Here, we show that mouse embryo fibroblasts (MEFs) obtained from mnk1 (-/-)/mnk2 (-/-) as well as mnk1 (-/-) and mnk2 (-/-) mice are sensitized to caspase-3 activation upon withdrawal of serum in comparison to wild-type cells. Caspase-3 cleavage occurs with all cells in the panel, but most rapidly and robustly in cells derived from mice lacking both MNK genes. Treatment of wild-type MEFs in the panel with a compound (CGP57380) that inhibits MNK1 and MNK2 sensitizes wild-type cells for serum-withdrawal induced apoptosis, suggesting that sensitization is due to loss of MNK function and not to a secondary event. Reintroduction of wild-type MNK1 in the double knockout MEFs results in decreased sensitivity to serum withdrawal that is not observed for wild-type MNK2, or the kinase dead variant. Our work identifies MNKs as kinases involved in anti-apoptotic signaling in response to serum withdrawal. [3] Chronic myeloid leukemia responds well to therapy targeting the oncogenic fusion protein BCR-ABL1 in chronic phase, but is resistant to treatment after it progresses to blast crisis (BC). BC is characterized by elevated β-catenin signaling in granulocyte macrophage progenitors (GMPs), which enables this population to function as leukemia stem cells (LSCs) and act as a reservoir for resistance. Because normal hematopoietic stem cells (HSCs) and LSCs depend on β-catenin signaling for self-renewal, strategies to specifically target BC will require identification of drugable factors capable of distinguishing between self-renewal in BC LSCs and normal HSCs. Here, we show that the MAP kinase interacting serine/threonine kinase (MNK)-eukaryotic translation initiation factor 4E (eIF4E) axis is overexpressed in BC GMPs but not normal HSCs, and that MNK kinase-dependent eIF4E phosphorylation at serine 209 activates β-catenin signaling in BC GMPs. Mechanistically, eIF4E overexpression and phosphorylation leads to increased β-catenin protein synthesis, whereas MNK-dependent eIF4E phosphorylation is required for nuclear translocation and activation of β-catenin. Accordingly, we found that a panel of small molecule MNK kinase inhibitors prevented eIF4E phosphorylation, β-catenin activation, and BC LSC function in vitro and in vivo. Our findings identify the MNK-eIF4E axis as a specific and critical regulator of BC self-renewal, and suggest that pharmacologic inhibition of the MNK kinases may be therapeutically useful in BC chronic myeloid leukemia. [4] |
| 分子式 |
C11H9FN6
|
|
|---|---|---|
| 分子量 |
244.23
|
|
| 精确质量 |
244.087
|
|
| 元素分析 |
C, 54.10; H, 3.71; F, 7.78; N, 34.41
|
|
| CAS号 |
522629-08-9
|
|
| 相关CAS号 |
|
|
| PubChem CID |
11644425
|
|
| 外观&性状 |
Light brown to brown solid powder
|
|
| 密度 |
1.6±0.1 g/cm3
|
|
| 沸点 |
541.6±50.0 °C at 760 mmHg
|
|
| 闪点 |
281.4±30.1 °C
|
|
| 蒸汽压 |
0.0±1.4 mmHg at 25°C
|
|
| 折射率 |
1.809
|
|
| LogP |
1.28
|
|
| tPSA |
92.51
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
18
|
|
| 分子复杂度/Complexity |
283
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
FC1C([H])=C([H])C(=C([H])C=1[H])N([H])C1=C2C(N([H])[H])=NC([H])=NC2=NN1[H]
|
|
| InChi Key |
UQPMANVRZYYQMD-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C11H9FN6/c12-6-1-3-7(4-2-6)16-11-8-9(13)14-5-15-10(8)17-18-11/h1-5H,(H4,13,14,15,16,17,18)
|
|
| 化学名 |
3-N-(4-fluorophenyl)-2H-pyrazolo[3,4-d]pyrimidine-3,4-diamine
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (10.24 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (10.24 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: 4% DMSO +30%PEG 300 +ddH2O: 10mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.0945 mL | 20.4725 mL | 40.9450 mL | |
| 5 mM | 0.8189 mL | 4.0945 mL | 8.1890 mL | |
| 10 mM | 0.4095 mL | 2.0473 mL | 4.0945 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|---|