| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg | |||
| 500mg | |||
| Other Sizes |
| 靶点 |
αvβ3 (IC50 = 4 nM, αvβ3-Vitronectin interaction); αvβ5 (IC50 = 79 nM, αvβ5-Vitronectin interaction); αvβ3 (IC50 = 0.61 nM); αvβ5 (IC50 = 8.4 nM); α5β1 (IC50 = 14.9 nM); STAT3
Cilengitide TFA (EMD 121974) specifically targets integrin receptors αVβ3 and αVβ5, with IC50 values of 4.1 nM (αVβ3) and 7.9 nM (αVβ5) for inhibiting ligand-receptor binding [1] Cilengitide TFA shows no significant binding to other integrins (e.g., αVβ6, α5β1, α2β1) at concentrations up to 1 μM [1] |
|---|---|
| 体外研究 (In Vitro) |
αvβ3 和 αvβ5 整合素受体的拮抗剂是西仑吉肽 (EMD 121974)。在分别评估人肺癌细胞系 UCLA-P3 或黑色素瘤 M21 细胞粘附的研究中,克仑吉肽可减少整合素介导的玻连蛋白结合,IC50 分别为 0.4 和 0.4 μM [1]。在体外,西仑吉肽在浓度高于 1 μM 时表现出浓度和时间依赖性细胞毒性作用 [2]。
在人类胶质母细胞瘤细胞系(U87MG、U251MG)中,Cilengitide TFA 抑制细胞增殖,U87MG 细胞的 IC50 为 8.3 μM,U251MG 细胞为 12.5 μM,20 μM 浓度处理 72 小时后细胞活力降低 60%-70% [2] - 10 μM Cilengitide TFA 通过阻断 αVβ3/αVβ5 介导的细胞与玻连蛋白粘附,使 U87MG 细胞的迁移能力降低 75%,侵袭能力降低 80% [2] - 在人类肉瘤细胞系(HT1080、SW982)中,15 μM Cilengitide TFA 增强美法仑诱导的细胞毒性,细胞活力降低 85%(美法仑单独处理仅降低 45%)[3] - 5 μM Cilengitide TFA 与贝洛替康在 U87MG 细胞中具有协同作用(协同指数 [CI] = 0.43),将贝洛替康的 IC50 从 0.3 μM 降至 0.09 μM [2] - 10 μM Cilengitide TFA 诱导 U87MG 细胞凋亡,48 小时后膜联蛋白 V 阳性细胞比例从 7% 升至 42%,伴随半胱天冬酶 -3 激活和 PARP 切割 [2] - 8 μM Cilengitide TFA 在人脐静脉内皮细胞(HUVEC)培养体系中抑制血管生成(内皮细胞成管)78%,阻断 αVβ3/αVβ5 依赖的血管出芽 [1] - Western blot 分析显示,Cilengitide TFA(5-15 μM)使胶质母细胞瘤和肉瘤细胞中 FAK(Tyr397)和 ERK1/2(Thr202/Tyr204)的磷酸化水平降低 65%-70% [2][3] |
| 体内研究 (In Vivo) |
将西来吉肽(10、50和250μg)每周3次腹腔注射给患有M21-L黑色素瘤的裸鼠;这些剂量被证明可以抑制肿瘤生长,同时缩小肿瘤体积(分别为 55%、75% 和 55%)。肿瘤重量(分别为 23%、38% 和 61%)和 89% [2]。在所研究的大鼠模型中,ILP单独与西仑吉肽联合给药、ILP与西仑吉肽联合美法仑、TNF或两者联合给药并不影响腹膜内施用的西仑吉肽的全身药代动力学。腹腔注射治疗10分钟后,西仑吉肽的全身水平达到约20μg/mL(约35μM),并在第一小时内持续上升至约40μg/mL(约70μM)。此后,赛利必肽血清水平的消除半衰期为 2.1 小时 [3]。
在 U87MG 人胶质母细胞瘤异种移植模型(nu/nu 小鼠)中,Cilengitide TFA 静脉给药(50 mg/kg,每日一次,连续 28 天)的肿瘤生长抑制率(TGI)达 68%,荷瘤小鼠中位生存期较溶媒组延长 50% [2] - 与贝洛替康(5 mg/kg,腹腔给药,每周一次,连续 4 周)联合使用时,Cilengitide TFA(50 mg/kg,静脉给药,每日一次)使 U87MG 异种移植模型的 TGI 提升至 86%,30% 的小鼠出现肿瘤退缩 [2] - 在大鼠肉瘤(R1 横纹肌肉瘤)孤立肢体灌注模型中,Cilengitide TFA(10 mg/kg,动脉内给药)联合美法仑(4 mg/kg)使肿瘤坏死率达 75%(美法仑单独处理仅 40%)[3] - Cilengitide TFA 处理组小鼠的肿瘤组织中,微血管密度降低 60%(较溶媒组),TUNEL 阳性凋亡细胞比例升至 35%(溶媒组为 8%)[1][2] |
| 酶活实验 |
整合素结合试验[Sci Rep. 2017 Jan 11;7:39805.]
整合素配体的活性和选择性通过固相结合试验确定,根据先前报道的方案,使用包被的细胞外基质蛋白和可溶性整合素。以下列化合物为内标:Cilengitide/西伦吉肽,c(RGDf(NMe)V) (αvβ3-0.54 nM, αvβ5-8 nM, α5β1-15.4 nM),线性肽RTDLDSLRT4 (αvβ6-33 nM;8 - 100 nM)和vαβtirofiban5 (IIbαβ3 - 1.2海里)。 用ecm蛋白(1)(每孔100 μL)在碳酸缓冲液(15 mM Na2CO3, 35 mM NaHCO3, pH 9.6)中在4°C下包被96孔平底ELISA板过夜。然后用pbs - t缓冲液(磷酸盐缓冲盐水/Tween20, 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, 0.01% Tween20, pH 7.4)洗涤每孔;3 × 200 μL),室温下用ts -b缓冲液(Tris-saline/BSA缓冲液;150μL /;20 mM Tris-HCl, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM MnCl2, pH 7.5, 1% BSA)。同时,将化合物和内标品以1:5的稀释步骤,从20 μM到6.4 nM,在另一个板上配制稀释系列。用PBS-T (200 μL)洗涤三次后,从B-G中每孔转移50 ul稀释系列。A孔填入100 ul tsb溶液(空白),H孔填入50 ul ts -b缓冲液。将人整合素(2)在ts -b缓冲液中的溶液50 ul转移到h -b孔中,rt孵育1 h, PBS-T缓冲液洗涤3次,然后加入一抗(3)(每孔100 μL)。rt孵育1 h后,用PBS-T洗涤3次。然后,在板中加入二次过氧化物酶标记抗体(4)(100 μL/孔),rt孵育1 h。PBS-T洗涤三次后,快速加入SeramunBlau (50 μL/孔,Seramun Diagnostic GmbH, Heidesee, Germany), rt孵育5 min。用3 M H2SO4 (50 μL/孔)停止反应,在450 nm处用平板仪测定吸光度。每个化合物的IC50分两份进行测试,用OriginPro 7.5G软件分析得到的抑制曲线。拐点表示IC50值。测定的IC50均参照内标活度。 整合素配体结合抑制实验:重组 αVβ3/αVβ5 整合素固定于微量滴定板。加入生物素化玻连蛋白(配体)和系列浓度的 Cilengitide TFA(0.1 nM 至 50 nM),37°C 孵育 60 分钟。链霉亲和素偶联试剂检测结合的配体,从抑制作用的剂量 - 反应曲线计算 IC50 值 [1] - 表面等离子体共振(SPR)结合测定:αVβ3/αVβ5 整合素固定于传感器芯片,系列浓度的 Cilengitide TFA(1 nM 至 30 nM)通过芯片,记录结合响应信号。推导解离常数(Kd)为 αVβ3(2.3 nM)和 αVβ5(5.7 nM)[1] |
| 细胞实验 |
蛋白质印迹分析[Bioengineered. 2022 Feb;13(2):4557-4572.]
细胞类型: B16 和 A375 细胞 测试浓度: 0、5、10 和 20 μg/mL 孵育持续时间: 12 小时 实验结果: 在浓度大于 5 μg/mL 时抑制 PD-L1 表达和 STAT3 磷酸化。 细胞凋亡分析[3] 细胞类型: B16 和 A375 细胞 测试浓度: 5 μg/mL 孵育时间:12小时 实验结果:B16和A375细胞的凋亡率分别为15.27%和14.89%。 抗增殖实验:胶质母细胞瘤、肉瘤或内皮细胞接种于 96 孔板(3×103 个细胞 / 孔),用系列浓度的 Cilengitide TFA(1 μM 至 50 μM)单独或与化疗药物联合处理 72 小时。基于四唑盐还原的比色法评估细胞活力,计算 IC50 值及协同指数 [1][2][3] - 迁移和侵袭实验:U87MG 或 HT1080 细胞接种于 Transwell 小室(迁移实验)或基质胶包被的 Transwell 小室(侵袭实验),并加入 Cilengitide TFA(5-20 μM)。24 小时后对迁移或侵袭的细胞进行染色计数 [2][3] - 凋亡实验:细胞经 Cilengitide TFA(10-15 μM)处理 48 小时后,用膜联蛋白 V-FITC 和碘化丙啶染色,流式细胞术分析。Western blot 检测半胱天冬酶 -3/PARP 切割 [2] - 血管生成实验:HUVEC 接种于基质胶包被的培养板,用 Cilengitide TFA(5-20 μM)处理 16 小时。相差显微镜观察成管情况,定量管腔分支数量 [1] - Western blot 分析:细胞用冰浴 RIPA 缓冲液裂解,蛋白经 SDS-PAGE 分离后转移至膜上,与抗 p-FAK、FAK、p-ERK1/2、ERK1/2、剪切型半胱天冬酶 -3、PARP 及 β- 肌动蛋白抗体孵育。化学发光法检测信号,密度计量法定量 [2][3] |
| 动物实验 |
Dissolved in PBS; 100μg; i.p. injection
Human glioblastoma xenografts U87 MG Animal/Disease Models: Nude mice bearing M21-L melanoma tumors[1] Doses: 10, 50, and 250 μg Route of Administration: Dosed ip three times per week Experimental Results: Demonstrated inhibition of tumor growth with a reduction in both tumor volume (55%, 75%, and 89%, respectively) and tumor weight (23%, 38%, and 61%, respectively), when compared to controls. Animal/Disease Models: Female C57BL/6 mice (6-8 weeks old) with B16 cells sc[Bioengineered. 2022 Feb;13(2):4557-4572.] Doses: 50 mg/kg; with or without 10 mg/kg Anti-PD1 monoclonal antibody or isotype control ip every 3 days; Route of Administration: intraperitoneal (ip)injection; daily Experimental Results: Downregulated the expression of PD-L1 via STAT3 pathway and diminished the expression of PD-L1. U87MG glioblastoma xenograft model: Female nu/nu mice (6-8 weeks old) were subcutaneously implanted with 5×106 U87MG cells. When tumors reached 100-150 mm3, mice were randomized into groups (n=8/group) and treated with: (1) vehicle (saline + 0.1% TFA) i.v., (2) Cilengitide TFA (50 mg/kg) i.v. once daily for 28 days, (3) Cilengitide TFA (50 mg/kg i.v. q.d.) + belotecan (5 mg/kg i.p. weekly for 4 weeks). Tumor volume and survival were monitored [2] - Rat sarcoma isolated limb perfusion model: Male Wistar rats (200-250 g) were implanted with R1 rhabdomyosarcoma cells in the hind limb. When tumors reached 1 cm3, rats were randomized into groups (n=6/group) and subjected to isolated limb perfusion with: (1) vehicle, (2) melphalan (4 mg/kg), (3) Cilengitide TFA (10 mg/kg) + melphalan (4 mg/kg). Tumor necrosis and volume were assessed 7 days post-perfusion [3] |
| 药代性质 (ADME/PK) |
In humans, intravenous administration of Cilengitide TFA (200 mg/m²) resulted in a Cmax of 12.8 μM, AUC0-∞ of 45.3 μM·h, and terminal half-life (t1/2) of 2.8 hours [1]
- In mice, intravenous administration of Cilengitide TFA (50 mg/kg) showed a clearance of 15.2 mL/min/kg, volume of distribution (Vss) of 0.8 L/kg, and t1/2 of 2.1 hours [1] - Cilengitide TFA exhibits low oral bioavailability (<5%) in animals and humans, requiring parenteral administration [1] - The drug is primarily excreted unchanged via the kidneys (78% of dose) within 24 hours of intravenous administration [1] - Human plasma protein binding of Cilengitide TFA is 25% at 10 μM concentration [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
In clinical trials, Cilengitide TFA showed manageable toxicity, with most common adverse events being mild-to-moderate fatigue (32%), headache (28%), and transient bleeding (18%) [1]
- In repeat-dose toxicity studies in mice (28 days, 30-100 mg/kg/day i.v.), Cilengitide TFA had a maximum tolerated dose (MTD) of 80 mg/kg/day, with dose-limiting toxicity (DLT) of mild thrombocytopenia (15% reduction) at 100 mg/kg/day [1] - Cilengitide TFA (50 mg/kg/day i.v. for 28 days) caused no significant histopathological abnormalities in liver, kidney, heart, or brain of mice [2] - No drug-drug interactions were observed when Cilengitide TFA was combined with belotecan or melphalan in preclinical studies [2][3] |
| 参考文献 |
|
| 其他信息 |
Background: Cilengitide, an antiangiogenic agent that inhibits the binding of integrins alpha(nu)beta(3) and alpha(nu)beta(5) to the extracellular matrix, was studied at two dose levels in cancer patients to determine the optimal biological dose. Patients and methods: The doses of cilengitide were 600 or 1200 mg/m(2) as a 1-h infusion twice weekly every 28 days. A novel dose escalation scheme was utilized that relied upon the biological activity rate. Results: Twenty patients received 50 courses of cilengitide with no dose-limiting toxic effects. The pharmacokinetic (PK) profile revealed a short elimination half-life of 4 h, supporting twice weekly dosing. Of the six soluble angiogenic molecules assessed, only E-selectin increased significantly from baseline. Analysis of tumor microvessel density and gene expression was not informative due to intrapatient tumor heterogeneity. Although several patients with evaluable tumor biopsy pairs did reveal posttreatment increases in tumor and endothelial cell apoptosis, these results did not reach statistical significance due to the aforementioned heterogeneity. Conclusions: Cilengitide is a well-tolerated antiangiogenic agent. The biomarkers chosen in this study underscore the difficulty in assessing the biological activity of antiangiogenic agents in the absence of validated biological assays.[1]
The prognosis of patients diagnosed with glioblastoma remains dismal in spite of the current concomitant chemoradiotherapy with temozolomide. In particular, the resistance to temozolomide appears to be the greatest obstacle to the treatment of glioblastoma. In the present study, we evaluated in vitro and in vivo the antitumor effects of combination therapy of cilengitide with belotecan, a camptothecin derivate, to treat experimental glioblastoma. The therapeutic effects of the drugs on the U87MG and U251MG human glioblastoma cell lines were assessed using in vitro cell viability and apoptosis assays. The combination treatment group with cilengitide and belotecan enhanced the cytotoxic effects to the glioblastoma cell lines and increased the apoptosis of the tumor cells compared to monotherapy with either drug alone in vitro. Nude mice with established U87MG glioblastoma were assigned to the following four groups: control, cilengitide, belotecan and combination treatment. The volume of tumors and length of survival were also measured. Animals in the combination therapy group demonstrated a significant reduction of tumor volume and an increase in survival (p < 0.05). Immunohistochemistry revealed a decrease in angiogenesis by cilengitide and an increase in apoptosis by cilengitide and belotecan in vivo. The combination therapy of cilengitide with belotecan presented more cytotoxic effects compared to the monotherapy of either drug in vitro and in vivo. This combination protocol may serve as an alternative treatment option for glioblastoma.[2] Isolated limb perfusion (ILP) with melphalan and tumor necrosis factor (TNF)-α is used to treat bulky, locally advanced melanoma and sarcoma. However, TNF toxicity suggests a need for better-tolerated drugs. Cilengitide (EMD 121974), a novel cyclic inhibitor of alpha-V integrins, has both anti-angiogenic and direct anti-tumor effects and is a possible alternative to TNF in ILP. In this study, rats bearing a hind limb soft tissue sarcoma underwent ILP using different combinations of melphalan, TNF and cilengitide in the perfusate. Further groups had intra-peritoneal (i.p.) injections of cilengitide or saline 2 hr before and 3 hr after ILP. A 77% response rate (RR) was seen in animals treated i.p. with cilengitide and perfused with melphalan plus cilengitide. The RR was 85% in animals treated i.p. with cilengitide and ILP using melphalan plus both TNF and cilengitide. Both RRs were significantly greater than those seen with melphalan or cilengitide alone. Histopathology showed that high RRs were accompanied by disruption of tumor vascular endothelium and tumor necrosis. Compared with ILP using melphalan alone, the addition of cilengitide resulted in a three to sevenfold increase in melphalan concentration in tumor but not in muscle in the perfused limb. Supportive in vitro studies indicate that cilengitide both inhibits tumor cell attachment and increases endothelial permeability. Since cilengitide has low toxicity, these data suggest the agent is a good alternative to TNF in the ILP setting.[3] Cilengitide TFA is a cyclic peptide inhibitor of αVβ3 and αVβ5 integrins, designed to block integrin-mediated cell adhesion, migration, and angiogenesis [1][2] The mechanism of action of Cilengitide TFA involves inhibiting integrin-ligand interactions, suppressing downstream FAK/ERK signaling, and reducing tumor cell proliferation, invasion, and vascular supply [1][2][3] Cilengitide TFA exhibits therapeutic potential for advanced solid tumors, particularly glioblastoma and sarcoma, and enhances the efficacy of chemotherapeutic agents (belotecan, melphalan) via synergistic cytotoxic and anti-angiogenic effects [2][3] Due to its low oral bioavailability, Cilengitide TFA is administered intravenously in clinical settings, with favorable safety profiles supporting combination therapy [1] |
| 分子式 |
C29H41F3N8O9
|
|
|---|---|---|
| 分子量 |
702.68
|
|
| 精确质量 |
702.295
|
|
| 元素分析 |
C, 55.09; H, 6.85; N, 19.04; O, 19.03
|
|
| CAS号 |
199807-35-7
|
|
| 相关CAS号 |
Cilengitide;188968-51-6; 199807-35-7 (TFA); 188969-00-8 (HCl)
|
|
| PubChem CID |
129626550
|
|
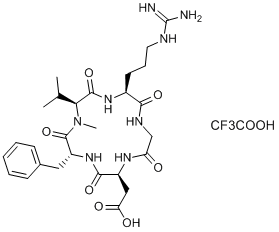
| 序列 |
cyclo[L-arginyl-glycyl-L-alpha-aspartyl-D-phenylalanyl-N-methyl-L-valyl] trifluoroacetic acid
|
|
| 短序列 |
cyclo[Arg-Gly-Asp-D-Phe-N(Me)Val].TFA
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| LogP |
1.111
|
|
| tPSA |
273.21
|
|
| 氢键供体(HBD)数目 |
8
|
|
| 氢键受体(HBA)数目 |
13
|
|
| 可旋转键数目(RBC) |
9
|
|
| 重原子数目 |
49
|
|
| 分子复杂度/Complexity |
1110
|
|
| 定义原子立体中心数目 |
4
|
|
| SMILES |
CC(C)[C@H]1C(=O)N[C@H](C(=O)NCC(=O)N[C@H](C(=O)N[C@@H](C(=O)N1C)CC2=CC=CC=C2)CC(=O)O)CCCN=C(N)N.C(=O)(C(F)(F)F)O
|
|
| InChi Key |
WHJCSACXAPYNTG-LOPTWHKWSA-N
|
|
| InChi Code |
InChI=1S/C27H40N8O7.C2HF3O2/c1-15(2)22-25(41)33-17(10-7-11-30-27(28)29)23(39)31-14-20(36)32-18(13-21(37)38)24(40)34-19(26(42)35(22)3)12-16-8-5-4-6-9-16;3-2(4,5)1(6)7/h4-6,8-9,15,17-19,22H,7,10-14H2,1-3H3,(H,31,39)(H,32,36)(H,33,41)(H,34,40)(H,37,38)(H4,28,29,30);(H,6,7)/t17-,18-,19+,22-;/m0./s1
|
|
| 化学名 |
2-[(2S,5R,8S,11S)-5-benzyl-11-[3-(diaminomethylideneamino)propyl]-7-methyl-3,6,9,12,15-pentaoxo-8-propan-2-yl-1,4,7,10,13-pentazacyclopentadec-2-yl]acetic acid;2,2,2-trifluoroacetic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 16.67 mg/mL (23.72 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
配方 2 中的溶解度: 30% Propylene glycol , 5% Tween 80 , 65% D5W: 30 mg/mL 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.4231 mL | 7.1156 mL | 14.2312 mL | |
| 5 mM | 0.2846 mL | 1.4231 mL | 2.8462 mL | |
| 10 mM | 0.1423 mL | 0.7116 mL | 1.4231 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。