| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
| 靶点 |
5-HT4 receptor ( EC50 = 140 nM ); hERG channel ( IC50 = 9.4 nM )
|
|---|---|
| 体外研究 (In Vitro) |
Cisapride (1-100 nM) 是一种有效的 hERG 膨胀剂,IC50 值为 9.4 nM[1]。 Cisapride (1-100 nM) 对 5-HT4 的输入有效,EC50 值为 140 nM[1]。西沙必利(0.3,1,3,10 和 30 μM)对 Kv4.3 的抑制作用呈剂量依赖性,IC50 值为 9.8 μM 在表达 Kv4.3 钾通道的 CHO 细胞[2]。
|
| 体内研究 (In Vivo) |
西沙必利 (0.1-1 mg/kg;注射,一次) 刺激清醒犬的胃窦和地下水运动[3]。 西沙必利 (2 mg/kg,(腹腔注射);4 mg/kg,(口腔);一次)与三硝基苯磺酸处理的端点在宏观特征、组织病理学特征、细胞因子谱和体重变化均无显着差异[4]。 动物模型:雄性Wistar大鼠用三硝基苯磺酸(TNBS)诱导的大鼠结肠炎[4] 剂量:2 mg/kg(腹腔注射); 4 mg/kg,(口服) 给药方式:2 mg/kg,腹腔注射; 4mg/kg,口服;结果:结肠炎大鼠出现严重、强烈的透壁炎症和弥漫性坏死、炎性肉芽肿和粘膜下中性粒细胞浸润。引起体重减轻。
迫切需要进行研究,以揭示旨在分析IBD治疗可能途径的靶点。由于血清素可能参与肠道炎症,5HT(4)受体在胃肠道功能中起着重要作用,因此研究5HT(3)受体在IBD发病机制中的作用将是有趣的。本研究旨在探讨5-羟色胺4受体激动剂西沙必利对三硝基苯磺酸(TNBS)诱导的大鼠结肠炎的影响。在大鼠使用TNBS诱导结肠炎两小时后,腹腔注射西沙必利(2mg/kg);4mg/kg,口服(p.o))和地塞米松(1mg/kg,i.p;2mg/kg,p.o)给药6天。此后,动物被安乐死;对远端结肠样本进行了宏观、组织学和生化评估以及ELISA检测。我们的数据显示,地塞米松治疗(i.p,p.o)显著降低了宏观和微观损伤以及生化标志物,但西沙必利(i.p或p.o)和TNBS治疗的大鼠在上述参数上没有显著差异。可以推断,由于TNBS引起的结肠炎严重程度很大(通过各种途径),西沙必利不能通过5HT(4)受体引起更多的结肠炎损伤。基于本研究,需要进一步的研究来调查5HT(4)受体在溃疡性结肠炎发病机制中的确切作用。[4] |
| 酶活实验 |
莫沙必利和西沙必利是具有5-羟色胺4受体激动剂活性的胃动力药物,已被广泛用于治疗各种胃肠道疾病。采用全细胞膜片钳技术研究了莫沙必利和西沙必利对克隆的Kv4.3通道在中国仓鼠卵巢细胞中稳定表达的影响。莫沙必利和西沙必利以浓度依赖的方式抑制Kv4.3,IC50值分别为15.2和9.8μM。莫沙必利以浓度依赖的方式加速Kv4.3的失活和活化速率,从而缩短达到峰值的时间。莫沙必利的缔合速率常数(k+1)和解离速率常数(k-1)分别为9.9μM(-1)s(-1)和151.3 s(-1)。K D(K-1/K+1)为16.2μM,与浓度反应曲线计算的IC50值相似。在通道打开的电压范围内观察到莫沙必利的电压依赖性抑制,但在所有Kv4.3通道都打开的电压区域内没有观察到。在莫沙必利存在下,Kv4.3的稳态激活和失活曲线均向超极化方向偏移。莫沙必利还显著加速了Kv4.3的闭合状态失活。莫沙必利产生使用依赖性抑制,这与Kv4.3失活后的缓慢恢复一致。M1和去甲西沙必利分别是莫沙必利和西沙必利的主要代谢产物,对Kv4.3的影响很小或没有影响。这些结果表明,莫沙必利通过在去极化过程中优先结合通道的开放状态和在亚阈值电位下加速封闭状态失活来抑制Kv4.3[2]
在受体结合研究中,莫沙必利抑制[3H]-GR113808与豚鼠纹状体5-HT4受体位点的结合,IC50值为113 nM[3]。 |
| 细胞实验 |
使用全细胞膜片钳技术在HEK293细胞中研究了三种5-羟色胺(4)激动剂西沙必利、莫沙必利和新发现的CJ-033466对人以太-a-go-go相关基因(hERG)通道的阻断作用。西沙必利被发现是最有效的hERG阻断剂。在受试化合物中,CJ-033466的hERG阻断活性和5-HT(4)激动作用之间的安全裕度最大。这表明与其他2种激动剂相比,CJ-033466的心律失常临床风险较低。因此,CJ-033466有可能成为一种比西沙必利和莫沙必利具有更高疗效和更低心脏风险的药物[1]。
|
| 动物实验 |
Male Wistar rats with trinitrobenzenesulfonic-acid-(TNBS) induced rat colitis
2 mg/kg (i.p.); 4 mg/kg, (oral administration) 2 mg/kg, intraperitoneal injection ; 4 mg/kg, oral administration; once Mosapride citrate is a new gastroprokinetic agent that enhances the upper GI motility by stimulating 5-hydroxytryptamine4 (5-HT4) receptors. The purpose of this study was to compare the effects of mosapride and the existing 5-HT4 receptor agonists on GI motility in conscious dogs and on various 5-HT4 receptor-mediated responses in vitro. In conscious dogs with force transducers implanted, mosapride (0.3-3 mg/kg i.v.) stimulated the antral motility without affecting the colonic motility. However, cisapride, zacopride and BIMU 8 (0. 1-1 mg/kg i.v.) stimulated both antral and colonic motility. The enhanced GI motility induced by mosapride or cisapride was antagonized by pretreatment with GR113808 (1 mg/kg bolus i.v., thereafter 1 mg/kg/hr infusion), a selective 5-HT4 receptor antagonist. In the receptor binding studies, mosapride inhibited [3H]-GR113808 binding to 5-HT4 receptor sites of guinea pig striatum with an IC50 value of 113 nM. In addition, mosapride caused relaxation of the carbachol-precontracted rat esophagus, enhanced the electrically evoked contractions of guinea pig ileum and evoked the contractions of guinea pig distal colon with EC50 values of 208, 73, and 3029 nM, respectively; this indicates that mosapride has a low affinity for colon than for the rest of the GI tract. In contrast, cisapride, zacopride or BIMU 8 had similar potencies in all preparations examined. In conclusion, these studies indicate that mosapride selectively stimulates upper GI motility in vivo and in vitro. These results also suggest heterogeneity of 5-HT4 receptors in the GI tract.[3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Cisapride is rapidly absorbed after oral administration, with an absolute bioavailability of 35-40%. The placental transfer of cisapride, a new prokinetic agent, was studied in a sheep model. The pharmacokinetics of cisapride were studied in the lamb, the pregnant ewe, and the fetus by obtaining blood samples from chronically implanted arterial catheters. Comparable pharmacokinetic parameters were found in the lamb and the adult sheep: half-life, 1.39-1.83 hr; total plasma clearance, 1998-2160 ml/kg/hr; AUC, 92.6-100.1 ng.hr/ml. Cisapride plasma concentrations after continuous infusion were predicted correctly based on the parameters obtained after IV bolus. There was a materno-fetal transfer of cisapride following a single IV bolus administered to the mother. Cisapride crossed the placenta within 5 min and equilibrated with maternal plasma within 20 to 30 min after dosing. The average fetal-to-maternal plasma concentration ratio was 0.71. The amniotic fluid also contained measurable amounts of cisapride. The protein binding of cisapride in maternal and fetal plasma is 89.0% and 88.4%, respectively; the free fraction is 4 times larger than in humans. Cisapride crosses the ovine placental barrier. The sheep placenta is less permeable than the human placenta, but the higher free fraction of cisapride facilitates placental transfer. Metabolism / Metabolites Hepatic. Extensively metabolized via cytochrome P450 3A4 enzyme. IPA COPYRIGHT: ASHP The metabolism of cisapride in vitro using Liver fractions of dogs, rabbits, and rats and the metabolites identified by high performance LC and by MS are described. Main bi otransformat i on routes were oxi dat i ve N-dealkylat i on at the pi peri di ne ni trogen and aron at i c hydroxylat i on at the fluorophenyl or at the benzami de moi ety. ENG ~21 nq~_~n_~. Hepatic. Extensively metabolized via cytochrome P450 3A4 enzyme. Half Life: 6-12 hours Biological Half-Life 6-12 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Cisapride was removed from the market in the United States by the U.S. Food and Drug Administration because of cardiac toxicity. Because of the low levels of cisapride in breastmilk, its use is acceptable in nursing mothers if it is required. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 97.5% Interactions Cispride has been reported to increase the rate of absorption of /alcohol or benzodiazepines/. Concurrent use /with anticholinergics or other medications with anticholinergic activity/ may antagonize the effects of cisapride on gastrointestinal motility. Cisapride accelerates the absorption of cimetidine and rantidine. Concurrent use of itraconazole, ketoconazole, or intravenous miconazole with cisapride is contraindicated; concurrent use may result in elevated plasma concentrations of cisapride through inhibition of the cytochrome p450 metabolic pathways by these antifungals; this has led to ventricular arrhythmias, including torsades des pointes, in patients taking cisapride and ketoconazole. For more Interactions (Complete) data for CISAPRIDE (8 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
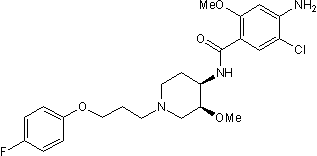
Cisapride is the amide resulting from formal condensation of 4-amino-5-chloro-2-methoxybenzoic acid with cis-1-[3-(4-fluorophenoxy)propyl]-3-methoxypiperidin-4-amine. It has been used (as its monohydrate or as its tartrate) for the treatment of gastro-oesophageal reflux disease and for non-ulcer dyspepsia, but its propensity to cause cardiac arrhythmias resulted in its complete withdrawal from many countries, including the U.K., and restrictions on its use elsewhere. It has a role as a serotonergic agonist, an anti-ulcer drug and a gastrointestinal drug. It is a member of piperidines, a member of benzamides, a member of monochlorobenzenes, a substituted aniline, an organofluorine compound and an aromatic ether.
In many countries (including Canada) cisapride has been either withdrawn or has had its indications limited due to reports about long QT syndrome due to cisapride, which predisposes to arrhythmias. The FDA issued a warning letter regarding this risk to health care professionals and patients. Cisapride is a substituted piperidinyl benzamide prokinetic agent. Cisapride facilitates release of acetylcholine from the myenteric plexus, resulting in increased gastrointestinal motility. In addition, cisapride has been found to act as a serotonin agonist, stimulating type 4 receptors, and a serotonin 5-HT3 receptor antagonist. (NCI) In many countries (including Canada) cisapride has been either withdrawn or has had its indications limited due to reports about long QT syndrome due to cisapride, which predisposes to arrhythmias. The FDA issued a warning letter regarding this risk to health care professionals and patients. A substituted benzamide used for its prokinetic properties. It is used in the management of gastroesophageal reflux disease, functional dyspepsia, and other disorders associated with impaired gastrointestinal motility. (Martindale The Extra Pharmacopoeia, 31st ed) See also: Cisapride Monohydrate (active moiety of). Drug Indication For the symptomatic treatment of adult patients with nocturnal heartburn due to gastroesophageal reflux disease. Mechanism of Action Cisapride acts through the stimulation of the serotonin 5-HT4 receptors which increases acetylcholine release in the enteric nervous system (specifically the myenteric plexus). This results in increased tone and amplitude of gastric (especially antral) contractions, relaxation of the pyloric sphincter and the duodenal bulb, and increased peristalsis of the duodenum and jejunum resulting in accelerated gastric emptying and intestinal transit. Cisapride exerts its effect by increasing the release of acetylcholine from the postganglionic nerve endings of the myenteric plexus. This release of acetylcholine increases esophageal activity and increases esophageal sphincter tone, thereby improving esophageal clearance and decreasing reflux of gastric and duodenal emptying as a result of increased gastric and duodenal contractility and antroduodenal coordination. Duodenogastric reflux is also decreased. Cisapride improves transit in both small and large bowel. Therapeutic Uses Anti-Ulcer Agents; Gastrointestinal Agents; Serotonin Agonists Cisapride is indicated for the symptomatic treatment of nocturnal (and daytime /Not included in US product labeling/) heartburn, and of esophagitis due to reflux and delayed gastric emptying. Treatment may continue for up to 8 weeks; however, tolerance to cisapride may develop at some point in therapy. /Included in US product labeling/ Cisapride is indicated in the treatment of gastroparesis, including idiopathic, diabetic, and intestinal pseudo-obstruction. Treatment may continue for up to 8 weeks; however, tolerance to cisapride may develop at some point in therapy. /NOT included in US product labeling/ ... Reduce the consumption of laxatives in patients who chronically abuse these agents. For more Therapeutic Uses (Complete) data for CISAPRIDE (7 total), please visit the HSDB record page. Drug Warnings Cisapride generally is well tolerated. Adverse effects on the GI tract and nervous system are most common and those most frequently requiring discontinuance of the drug(usually because of intolerable diarrhea and/or abdominal pain). The most common adverse GI effects (e.g., diarrhea) are extensions of the drug's pharmacologic activity. Because of differences in the pharmacologic profiles of the drugs, adverse nervous system effects are less common with cisapride than with metoclopramide whereas diarrhea is more common with cisapride. In adults receiving cisapride for motility disorder in US placebo-controlled clinical trials, including those with gastroesophageal reflux disease, the most frequent adverse effects of cisapride were headache, diarrhea, abdominal pain, nausea, constipation, and rhinitis. The frequency of diarrhea, abdominal pain, constipation, flatulence, and rhinitis appears to be dose dependent, occurring more frequently in patients receiving oral cisapride 20 mg 4 times daily than in those receiving 10 mg 4 times daily. Many adverse effects reported with cisapride occurred at a frequency similar to that associated with placebo, and a causal relationship to the drug often could not be established. Dehydration was reported in more than 1% of patients receiving cisapride in controlled clinical trials. Limited evidence indicates that cisapride does not adversely affect glycemic control in insulin-dependent (type I) diabetic patients with delayed gastric emptying. Viral infection occurred in about 4% of patients receiving cisapride in controlled clinical trails and required discontinuance of the drug in 0.2% of patients. Fever was reported in about 2% of patients receiving cisapride in controlled clinical trials and required discontinuance in 0.1%. For more Drug Warnings (Complete) data for CISAPRIDE (6 total), please visit the HSDB record page. Pharmacodynamics Cisapride is a parasympathomimetic which acts as a serotonin 5-HT4 agonist; upon activation of the receptor signaling pathway, cisapride promotes the release of acetylcholine neurotransmitters in the enteric nervous system. Cisapride stimulates motility of the upper gastrointestinal tract without stimulating gastric, biliary, or pancreatic secretions. Cisapride increases the tone and amplitude of gastric (especially antral) contractions, relaxes the pyloric sphincter and the duodenal bulb, and increases peristalsis of the duodenum and jejunum resulting in accelerated gastric emptying and intestinal transit. It increases the resting tone of the lower esophageal sphincter. It has little, if any, effect on the motility of the colon or gallbladder. Cisapride does not induce muscarinic or nicotinic receptor stimulation, nor does it inhibit acetylcholinesterase activity. |
| 分子式 |
C23H29CLFN3O4
|
|---|---|
| 分子量 |
465.95
|
| 精确质量 |
465.183
|
| 元素分析 |
C, 59.29; H, 6.27; Cl, 7.61; F, 4.08; N, 9.02; O, 13.73
|
| CAS号 |
81098-60-4
|
| 相关CAS号 |
Cisapride monohydrate; 260779-88-2; 81098-60-4; 189888-25-3 (tartrate)
|
| PubChem CID |
6917698
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
605.4±55.0 °C at 760 mmHg
|
| 熔点 |
107 - 111ºC
|
| 闪点 |
319.9±31.5 °C
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
| 折射率 |
1.593
|
| LogP |
3.12
|
| tPSA |
86.05
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
32
|
| 分子复杂度/Complexity |
581
|
| 定义原子立体中心数目 |
2
|
| SMILES |
C(C1C=C(Cl)C(N)=CC=1OC)(=O)N[C@@H]1CCN(CCCOC2C=CC(F)=CC=2)C[C@@H]1OC
|
| InChi Key |
DCSUBABJRXZOMT-IRLDBZIGSA-N
|
| InChi Code |
InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29)/t20-,22+/m1/s1
|
| 化学名 |
4-amino-5-chloro-N-[(3S,4R)-1-[3-(4-fluorophenoxy)propyl]-3-methoxypiperidin-4-yl]-2-methoxybenzamide
|
| 别名 |
R51619; R 51619; R-51619; Cisaprida; Cisapridum; (+-)-Cisapride; CHEBI:3720; Kaudalit; Prepulsid; Kinestase; Pridesia; Presid; Propulsid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 93~100 mg/mL (199.6~214.6 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.37 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.37 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1462 mL | 10.7308 mL | 21.4615 mL | |
| 5 mM | 0.4292 mL | 2.1462 mL | 4.2923 mL | |
| 10 mM | 0.2146 mL | 1.0731 mL | 2.1462 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01281566 | Terminated | Drug: Placebo Drug: Cisapride |
Infant, Premature Infant, Newborn |
Johnson & Johnson Pharmaceutical Research & Development, L.L.C. |
March 2003 | Phase 4 |
| NCT01286090 | Terminated | Drug: Placebo Drug: Cisapride |
Gastroparesis Diabetes Mellitus |
Johnson & Johnson Pharmaceutical Research & Development, L.L.C. |
July 2003 | Phase 4 |
| NCT01281540 | Terminated | Drug: Placebo Drug: Cisapride |
Gastroparesis | Johnson & Johnson Pharmaceutical Research & Development, L.L.C. |
May 2003 | Phase 4 |