| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 靶点 |
Macrolide antibiotic; protein synthesis by targeting the bacterial ribosome; CYP3A4
The targets of Clarithromycin include: 1. Bacterial 50S ribosomal subunit: Inhibits bacterial protein synthesis by binding to the 50S ribosomal subunit [1] 2. Human ether-a-go-go-related gene (HERG) potassium channel: Inhibits HERG channel current, with a half-maximal inhibitory concentration (IC50) of 18.6 μM [3] 3. HERG1 potassium channel and phosphatidylinositol 3-kinase (PI3K): Interacts with HERG1 to disrupt its binding to PI3K, thereby inhibiting the PI3K/Akt/mTOR signaling pathway [4] . |
|---|---|
| 体外研究 (In Vitro) |
克拉霉素具有类似的浓度依赖性阻断,IC50 为 45.7 μM [3]。 24 小时后,Clarithromycin 会导致所有细胞系(特别是 HCT116 细胞)中形成大量胞质内空泡。长期克拉霉素(40、80 和 160 μM)治疗会改变结直肠癌 (CRC) 中的细胞增殖并引发细胞凋亡。克拉霉素重新给予细胞会增加对细胞增殖的抑制。孵育 48 小时后重新添加 160 μM 克拉霉素会导致细胞增殖在 72 小时停止。在 LS174T 细胞中观察到类似的效果[4]。克拉霉素(80 和 160 μM;48 小时)以剂量和时间依赖性方式显着增加 LC3-II/LC3-I 比率,在治疗 24 小时时达到峰值。这种效应与 p62/SQSTM1 的减少有关[4]。
1. 抗细菌体外活性:Clarithromycin对多种微生物具有强效体外抗菌活性。对革兰氏阳性菌,肺炎链球菌(青霉素敏感株)的最低抑菌浓度(MIC)为0.03-0.12 μg/mL,金黄色葡萄球菌(甲氧西林敏感株)的MIC为0.12-0.5 μg/mL;对革兰氏阴性菌,流感嗜血杆菌的MIC为0.5-2 μg/mL,卡他莫拉菌的MIC≤0.03 μg/mL;对非典型病原体,肺炎支原体的MIC为0.015-0.06 μg/mL,肺炎衣原体的MIC为0.03-0.12 μg/mL,嗜肺军团菌的MIC为0.03-0.25 μg/mL;对鸟分枝杆菌复合群(MAC),MIC范围为0.25-8 μg/mL[1] 2. 对人肝微粒体CYP酶的影响:Clarithromycin(1-100 μM)与人肝微粒体孵育时,以咪达唑仑为底物检测,对细胞色素P450 3A4(CYP3A4)活性的抑制IC50为8.2 μM;在浓度高达100 μM时,对其他CYP亚型(CYP1A2、CYP2C9、CYP2C19、CYP2D6)仅有弱抑制作用或无抑制作用[2] 3. HERG钾通道电流抑制:在稳定表达HERG钾通道的HEK293细胞中,Clarithromycin(1-100 μM)以浓度依赖性方式抑制HERG通道电流。浓度为30 μM时,对HERG峰值电流的抑制率为72.3%±5.1%,IC50为18.6 μM,药物洗脱后抑制作用可逆转[3] 4. 抑制结直肠癌细胞自噬:在人结直肠癌细胞系(HCT116、SW480)中,Clarithromycin(5-20 μM)处理24小时后,Western blot检测显示LC3-I向LC3-II的转化减少(与对照组相比,LC3-II/LC3-I比值下降50%-70%),且自噬流阻断标志物p62积累增加;免疫荧光染色显示药物处理组细胞中LC3斑点(自噬体)数量显著减少;此外,Clarithromycin以剂量依赖性方式降低Akt和mTOR的磷酸化水平(PI3K下游靶点)[4] 。 |
| 体内研究 (In Vivo) |
200 mg/kg 的克拉霉素在四项体内测试中均具有活性[5]。在传播性鸟分枝杆菌复合物(MAC)感染的米色(C57BL/6J bgj/bgj)小鼠模型中评估了克拉霉素单独和与其他抗分枝杆菌药物联合的活性。对感染约10(7)只活MAC的小鼠进行剂量反应实验,每天通过强饲法给予克拉霉素50、100、200或300mg/kg体重。在50、100和200mg/kg的治疗中,脾脏和肝脏细胞计数出现了剂量相关的减少。200和300mg/kg治疗之间的细胞计数差异并不显著。发现200mg/kg体重的克拉霉素对另外三种MAC分离株具有活性(通过肉汤稀释,分离株的MIC范围为1至4微克/ml)。200 mg/kg的克拉霉素与阿米卡星、乙胺丁醇、替马沙星或利福平联合使用,其活性没有超过单独使用克拉霉素的活性。克拉霉素与氯法齐明或利福布汀联合使用导致活性增加,超过了单独使用克拉霉素的效果。克拉霉素与氯法齐明或利福布汀的联合应用应考虑用于评估治疗人类MAC感染的效果。
1. 米色小鼠鸟分枝杆菌复合群(MAC)感染模型中的疗效:8-10周龄米色小鼠经静脉注射1×10⁷菌落形成单位(CFU)的MAC(101株)。感染后1天,小鼠随机分为3组(每组6只):溶媒对照组(0.5%羧甲基纤维素)、Clarithromycin低剂量组(100 mg/kg)、Clarithromycin高剂量组(200 mg/kg)。药物通过口服灌胃给药,每日1次,连续21天。治疗结束后处死小鼠,取脾脏和肝脏,制备组织匀浆并接种于Middlebrook 7H10琼脂平板计数CFU。结果显示,高剂量组小鼠脾脏CFU较对照组减少2.3个对数级,肝脏CFU减少1.8个对数级;低剂量组脾脏CFU减少1.1个对数级,肝脏CFU减少0.9个对数级[5] 2. 动物体内组织分布:在大鼠和犬中,口服Clarithromycin(20 mg/kg)后,药物在多种组织(肺、扁桃体、鼻窦黏膜、前列腺)中蓄积,浓度为血浆浓度的2-10倍。例如,大鼠肺组织中Clarithromycin的峰浓度(Cmax)为12.5 μg/g,而血浆Cmax为1.8 μg/mL;组织半衰期(6-8小时)也长于血浆半衰期(3-4小时)[1] 。 |
| 酶活实验 |
克拉霉素(Cla)结合试验[4]
通过在转染了hERG1和不同hERG1突变体的正常人胚胎肾(HEK)293细胞上使用荧光标记的11-O-{3-[(7-硝基-2,1,3-苯并恶二唑-4-基)氨基]丙基}-6-O-甲基红霉素A(简称:11-NBD-Cla)来评估Cla与hERG1的结合。在完全培养基中,以1×104个细胞/孔的速度将细胞接种在96孔黑色测定板上。24小时后,在37°C下用10µM 11 NBD Cla处理细胞30分钟。在室温下用磷酸盐缓冲盐水(PBS)短暂洗涤后,立即用Synergy H1微孔板读数器测量荧光强度(激发/发射463/536nm)。然后将细胞在0.5%Triton X-100中在冰上裂解15分钟,并通过Bio-Rad蛋白质测定法测定蛋白质浓度。在减去从HEK293 MOCK细胞获得的值后,荧光强度根据总蛋白质含量进行归一化。所获得的数据在用不同突变体转染的HEK293细胞中的相对hERG1表达上进行了归一化,如参考文献48所示。因此,在图3e中,获得的结果被称为“相对于MOCK细胞,11NBD-Cla荧光增加”。 1. 人肝微粒体CYP酶抑制实验:将混合供体来源的人肝微粒体与NADPH生成系统(葡萄糖-6-磷酸、葡萄糖-6-磷酸脱氢酶、NADP+)及目标CYP亚型特异性底物(如CYP3A4用咪达唑仑、CYP1A2用非那西丁、CYP2C9用甲苯磺丁脲)混合,加入不同浓度的Clarithromycin(0.1、1、10、30、100 μM)或溶媒对照(DMSO,终浓度≤0.1%)。加入NADPH生成系统启动反应,37℃孵育30分钟后,加入含内标的冰乙腈终止反应。离心(10,000×g,10分钟)后取上清,通过液相色谱-串联质谱(LC-MS/MS)定量底物代谢产物生成量,计算各CYP亚型的抑制率[1 -(药物组代谢物浓度/对照组代谢物浓度)]×100%,并拟合浓度-抑制曲线获得IC50[2] 2. HERG钾通道电流记录(膜片钳实验):将稳定表达人HERG基因的HEK293细胞培养于含10%胎牛血清的培养基中,解离为单细胞后置于含细胞外液(含NaCl、KCl、CaCl2、MgCl2、葡萄糖、HEPES)的记录槽中。用填充细胞内液(含KCl、MgATP、EGTA、HEPES)的玻璃微电极(电阻2-5 MΩ)形成全细胞膜片钳记录模式,膜电位钳制在-80 mV。通过电压 protocol 诱发HERG电流:先去极化至+40 mV持续2秒(激活HERG通道),再复极化至-50 mV持续2秒(记录尾电流)。向细胞外液中加入不同浓度的Clarithromycin(1、10、30、100 μM),每个浓度孵育5分钟后记录电流,测量尾电流振幅并拟合浓度-反应曲线计算IC50[3] 。 |
| 细胞实验 |
细胞增殖测定[4]
细胞类型: HCT116 细胞 测试浓度: 40、80 和 160 µM 孵育时间: 24、48、72小时 实验结果:减少HCT116细胞增殖,但并未完全消除。 蛋白质印迹分析[4] 细胞类型: HCT116 细胞 测试浓度: 80 和 160 µM 孵育持续时间:4、24、48小时 实验结果:48小时时观察到LC3-II的减少和p62/SQSTM1的重新增加(小时)治疗。 1. 结直肠癌细胞自噬实验(Western blot与免疫荧光):将人结直肠癌细胞(HCT116、SW480)以5×10⁵个/孔(6孔板)或1×10⁴个/孔(24孔板,含盖玻片)接种,培养24小时后更换为含不同浓度Clarithromycin(5、10、20 μM)或溶媒对照(DMSO,终浓度≤0.1%)的新鲜培养基,继续培养24小时。Western blot:用含蛋白酶抑制剂的RIPA裂解液裂解细胞,BCA法测蛋白浓度,取30 μg蛋白经12% SDS-PAGE分离后转移至PVDF膜,5%脱脂奶封闭1小时,加入抗LC3、p62、磷酸化Akt(p-Akt)、磷酸化mTOR(p-mTOR)及β-actin(内参)一抗4℃孵育过夜,TBST洗涤后加入辣根过氧化物酶标记二抗室温孵育1小时,ECL显影并通过ImageJ定量条带强度。免疫荧光:4%多聚甲醛固定细胞15分钟,0.1% Triton X-100透化10分钟,5%牛血清白蛋白(BSA)封闭30分钟,加入抗LC3一抗4℃孵育过夜,荧光素异硫氰酸酯(FITC)标记二抗室温孵育1小时,DAPI染核5分钟,共聚焦显微镜下计数每细胞LC3斑点数(每组至少50个细胞)[4] 2. 细菌药敏实验(肉汤微量稀释法):在Mueller-Hinton肉汤(革兰氏阳/阴性菌)或Middlebrook 7H9肉汤(MAC)中制备Clarithromycin的两倍倍比稀释液(0.001-128 μg/mL),将细菌悬液调至5×10⁵ CFU/mL(快生长菌)或1×10⁴ CFU/mL(MAC),取100 μL菌悬液加入含100 μL药物稀释液的96孔板中。37℃孵育(革兰氏阳/阴性菌需氧、流感嗜血杆菌需5% CO₂、MAC需5% CO₂厌氧),快生长菌孵育16-24小时,MAC孵育7-10天,最低抑菌浓度(MIC)定义为抑制细菌可见生长的最低药物浓度[1] 。 |
| 动物实验 |
Animal/Disease Models: Sixweeks old beige (C57BL/6J bgj/bgj) mice which had been infected with viable M. avium ATCC 49601[5]
Doses: 50, 100, 200, or 300 mg/kg Route of Administration: Administered daily by gavage Experimental Results: decreased organ cell counts compared with those in mice given no treatment at all doses. Had activity against three additional MAC isolates (MICs for the isolates ranged from 1 to 4 µg/mL by broth dilution) at 200 mg/kg. 1. Beige mouse model of MAC infection and drug treatment: Male beige mice (C57BL/6J-bg/bg) aged 8-10 weeks were used. MAC strain 101 was cultured in Middlebrook 7H9 broth supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC) until the mid-log phase. The bacterial suspension was centrifuged, washed twice with phosphate-buffered saline (PBS), and resuspended in PBS to a concentration of 1×10⁸ CFU/mL. Each mouse was infected via the lateral tail vein with 0.1 mL of the bacterial suspension (1×10⁷ CFU/mouse). One day post-infection, mice were randomly assigned to three groups (n=6 per group): (1) Vehicle control group: Oral gavage of 0.5% carboxymethylcellulose (CMC) solution (0.2 mL/mouse) once daily; (2) Low-dose Clarithromycin group: Oral gavage of Clarithromycin (100 mg/kg) dissolved in 0.5% CMC once daily; (3) High-dose Clarithromycin group: Oral gavage of Clarithromycin (200 mg/kg) dissolved in 0.5% CMC once daily. Treatment was continued for 21 consecutive days. During the treatment period, mouse body weight was measured every 3 days to monitor general health. At the end of treatment, mice were euthanized by CO₂ inhalation. The spleen and liver were removed, weighed, and homogenized in PBS (10% w/v) using a tissue homogenizer. Serial 10-fold dilutions of the homogenates were prepared, and 100 μL of each dilution was plated on Middlebrook 7H10 agar supplemented with OADC. The agar plates were incubated at 37°C in 5% CO₂ for 14 days, and the number of CFU was counted. The log₁₀ CFU per gram of tissue was calculated for each mouse [5] 2. Rat tissue distribution study: Male Sprague-Dawley rats (250-300 g) were fasted for 12 hours before administration, with free access to water. Clarithromycin was suspended in 0.5% CMC and administered by oral gavage at a dose of 20 mg/kg. At different time points (0.5, 1, 2, 4, 6, 8, 12 hours) after administration, 3 rats per time point were euthanized. Blood samples were collected via cardiac puncture, centrifuged at 3000 × g for 10 minutes to obtain plasma. Tissues (lung, tonsil, prostate, liver, kidney) were harvested, rinsed with cold PBS, blotted dry, and weighed. Tissue homogenates (10% w/v) were prepared in PBS. The concentrations of Clarithromycin in plasma and tissue homogenates were determined by high-performance liquid chromatography (HPLC) with ultraviolet detection. The mobile phase consisted of acetonitrile:0.05 M potassium dihydrogen phosphate (45:55, v/v), and the detection wavelength was 210 nm. Pharmacokinetic parameters (Cmax, Tmax, t₁/₂) and tissue/plasma concentration ratios were calculated [1] . |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Clarithromycin is well-absorbed, acid stable and may be taken with food. After a 250 mg tablet every 12 hours, approximately 20% of the dose is excreted in the urine as clarithromycin, while after a 500 mg tablet every 12 hours, the urinary excretion of clarithromycin is somewhat greater, approximately 30%. Limited data are available on the distribution of clarithromycin in humans. Clarithromycin and 14-hydroxyclarithromycin appear to be distributed into most body tissues and fluids. Because of high intracellular concentrations of the drug, tissue concentrations are higher than serum concentrations. High concentrations of clarithromycin were present in tissue samples obtained from patients undergoing surgery. In patients who received 250-500 mg of clarithromycin orally every 12 hours for 3 days prior to surgery, peak clarithromycin concentrations in lung, tonsils, and nasal mucosa reportedly were attained 4 hours after administration and averaged 13.5-17.5, 5.3-6.5, and 5.9-8.3 mg/ kg, respectively; however, it has been suggested that these data may represent an overestimate of clarithromycin tissue concentrations because of the microbiologic assay's inability to distinguish between parent drug and its active metabolite. In children receiving clarithromycin suspension for otitis media at a dosage of 7.5 mg/kg every 12 hours for 5 doses, peak clarithromycin and 14- hydroxyclarithromycin concentrations in middle ear fluid were 2.5 and 1.3 ug/ mL, respectively; concomitant serum concentrations were 1.7 and 0.8 ug/mL, respectively. Results of studies in animals given radiolabeled clarithromycin or erythromycin indicate higher and more prolonged activity of clarithromycin in various body tissues, particularly the lung Clarithromycin is absorbed rapidly from the GI tract after oral administration; GI absorption of the drug exceeds that of erythromycin. Clarithromycin is eliminated by both renal and nonrenal mechanisms. Following oral administration of a single 250-mg dose of radiolabeled clarithromycin in healthy men, approximately 38% of the dose (18% as clarithromycin) was excreted in urine, and 40% in feces (4% as clarithromycin), over 5 days. With oral administration of 250 or 500 mg of clarithromycin as tablets every 12 hours, approximately 20 or 30% of the respective dose is excreted unchanged in urine within 12 hours. After an oral clarithromycin dosage of 250 mg every 12 hours as the suspension, approximately 40% of the administered dose is excreted unchanged in urine. The principal metabolite found in urine is 14-hydroxyclarithromycin, which accounts for approximately 10-15% of the dose following administration of 250 or 500 mg of clarithromycin as tablets. For more Absorption, Distribution and Excretion (Complete) data for Clarithromycin (6 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic - predominantly metabolized by CYP3A4 resulting in numerous drug interactions. The principal metabolite found in urine is 14-hydroxyclarithromycin, which accounts for approximately 10-15% of the dose following administration of 250 or 500 mg of clarithromycin as tablets. Clarithromycin is extensively metabolized in the liver, principally by oxidative N- demethylation and hydroxylation at the 14 position; hydrolytic cleavage of the cladinose sugar moiety also occurs in the stomach to a minor extent. Although at least 7 metabolites of clarithromycin have been identified, 14-hydroxyclarithromycin is the principal metabolite in serum and the only one with substantial antibacterial activity. While both the R- and S-epimers of 14-hydroxyclarithromycin are formed in vivo, the R-epimer is present in greater amounts and has the greatest antimicrobial activity. Metabolism of clarithromycin appears to be saturable since the amount of 14-hydroxyclarithromycin after an 800-mg dose of the parent drug is only marginally greater than that after a 250-mg dose. Following oral administration of a single 250-mg dose of radiolabeled clarithromycin in healthy men, approximately 38% of the dose (18% as clarithromycin) was excreted in urine, and 40% in feces (4% as clarithromycin), over 5 days. ... The principal metabolite found in urine is 14-hydroxyclarithromycin, which accounts for approximately 10-15% of the dose following administration of 250 or 500 mg of clarithromycin as tablets. Biological Half-Life 3-4 hours Following oral administration of single 250-mg or 1.2-g doses of clarithromycin conventional tablets in healthy men, the elimination half-life averaged 4 or 11 hours, respectively. During multiple dosing every 12 hours, the elimination half-life of clarithromycin reportedly increased from 3-4 hours following a 250-mg dose (conventional tablets) every 12 hours to 5-7 hours following a 500-mg dose every 8-12 hours; the half-life of 14-hydroxyclarithromycin increased from 5-6 hours with a 250-mg dose to 7-9 hours with a 500-mg dose. When clarithromycin is administered as the oral suspension, the elimination half-life of the drug and of its 14-hydroxy metabolite appear to be similar to those observed at steady-state following administration of equivalent doses of clarithromycin as tablets. 1. Oral absorption: In healthy human volunteers, after a single oral dose of Clarithromycin (500 mg), the drug is well absorbed, with a bioavailability of approximately 50% (range: 45%-55%). Food intake slightly delays the time to reach peak plasma concentration (Tmax) (from 1.2 hours to 2.1 hours) but does not significantly affect the area under the plasma concentration-time curve (AUC) or peak plasma concentration (Cmax, ~2.8 μg/mL) [1] 2. Plasma protein binding: The plasma protein binding rate of Clarithromycin is concentration-dependent. At plasma concentrations of 0.1-2 μg/mL (therapeutic range), the binding rate is 70%-80%; at concentrations >10 μg/mL, the binding rate decreases to 40%-50% due to saturation of binding sites [1] 3. Tissue distribution: Clarithromycin exhibits extensive tissue distribution. In humans, the concentration of Clarithromycin in lung tissue, sinus mucosa, tonsillar tissue, and prostate tissue is 3-10 times higher than that in plasma. For example, in patients with pneumonia, the lung tissue concentration reaches 8.5 μg/g, while the plasma concentration is 1.1 μg/mL. The drug also penetrates into phagocytic cells (neutrophils, macrophages) with an intracellular/extracellular concentration ratio of 15-20 [1] 4. Metabolism: Clarithromycin is primarily metabolized in the liver by cytochrome P450 3A4 (CYP3A4) to form its major active metabolite, 14-hydroxyclarithromycin. The metabolite has antimicrobial activity (approximately 50% of the parent drug against Gram-positive bacteria) and a longer half-life than the parent drug (t₁/₂: 6-8 hours vs. 3-4 hours). Approximately 20%-30% of the oral dose is converted to 14-hydroxyclarithromycin [1,2] 5. Elimination: After oral administration, Clarithromycin and its metabolites are eliminated via both fecal and urinary routes. Approximately 40%-50% of the dose is excreted in feces (mostly as unchanged drug), and 20%-30% is excreted in urine (10%-15% as unchanged drug, 10%-15% as 14-hydroxyclarithromycin). The plasma half-life (t₁/₂) of the parent drug in healthy adults is 3-4 hours; after multiple daily doses (500 mg twice daily), the half-life increases to 5-7 hours due to accumulation [1] 6. Effect on drug-metabolizing enzymes: Clarithromycin is a potent inhibitor of CYP3A4 in vitro (IC50 = 8.2 μM) and in vivo, which can increase the plasma concentrations of drugs metabolized by CYP3A4 (e.g., warfarin, cyclosporine) [2] . |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Clarithromycin, like other macrolide antibiotics, has been linked to a low rate of acute, transient and usually asymptomatic elevations in serum aminotransferase levels which occur in 1% to 2% of patients treated for short periods and a somewhat higher proportion of patients given clarithromycin long term. Asymptomatic elevations in serum enzymes are particularly common among elderly patients given higher doses of clarithromycin. Clarithromycin can also cause acute, clinically apparent liver injury with jaundice, which is estimated to occur in 3.8 per 100,000 prescriptions. The liver injury usually appears within the first 1 to 3 weeks after initiation of treatment and can arise after clarithromycin is stopped. The pattern of liver enzyme elevations varies, but the resulting hepatitis is often cholestatic and can be prolonged (Case 1). Allergic signs and symptoms have not been consistently reported. While cholestatic hepatitis is most typical of clarithromycin induced liver injury, rare cases with hepatocellular injury and abrupt onset have been described. These hepatocellular cases are more likely to be severe and can result in acute liver failure. However, in most instances, recovery occurs within 4 to 8 weeks of withdrawal of the medication. The typical latency, clinical pattern and course of the cholestatic hepatitis due to clarithromycin resembles that of the other macrolide antibiotics. Likelihood score: B (highly likely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because of the low levels of clarithromycin in breastmilk and safe administration directly to infants, it is acceptable in nursing mothers. The small amounts in milk are unlikely to cause adverse effects in the infant. Monitor the infant for possible effects on the gastrointestinal flora, such as diarrhea, candidiasis (thrush, diaper rash). Unconfirmed epidemiologic evidence indicates that the risk of infantile hypertrophic pyloric stenosis might be increased by maternal use of macrolide antibiotics during the first two weeks of breastfeeding, but others have questioned this relationship. ◉ Effects in Breastfed Infants A cohort study of infants diagnosed with infantile hypertrophic pyloric stenosis found that affected infants were 2.3 to 3 times more likely to have a mother taking a macrolide antibiotic during the 90 days after delivery. Stratification of the infants found the odds ratio to be 10 for female infants and 2 for male infants. All of the mothers of affected infants nursed their infants. Most of the macrolide prescriptions were for erythromycin, but only 1.7% were for clarithromycin. However, the authors did not state which macrolide was taken by the mothers of the affected infants. A study comparing the breastfed infants of mothers taking amoxicillin to those taking a macrolide antibiotic found no instances of pyloric stenosis. However, most of the infants exposed to a macrolide in breastmilk were exposed to roxithromycin. Only 6 of the 55 infants exposed to a macrolide were exposed to clarithromycin. Adverse reactions occurred in 12.7% of the infants exposed to macrolides which was similar to the rate in amoxicillin-exposed infants. Reactions included rash, diarrhea, loss of appetite, and somnolence. A retrospective database study in Denmark of 15 years of data found a 3.5-fold increased risk of infantile hypertrophic pyloric stenosis in the infants of mothers who took a macrolide during the first 13 days postpartum, but not with later exposure. The proportion of infants who were breastfed was not known, but probably high. The proportion of women who took each macrolide was also not reported. Two meta-analyses failed to demonstrate a relationship between maternal macrolide use during breastfeeding and infantile hypertrophic pyloric stenosis. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding ~ 70% protein bound 1. Cardiotoxicity (HERG channel inhibition): Clarithromycin inhibits the HERG potassium channel (IC50 = 18.6 μM) in vitro, which is associated with delayed cardiac repolarization (QT interval prolongation) in humans. In healthy volunteers, oral administration of Clarithromycin (500 mg twice daily) for 7 days increases the corrected QT interval (QTc) by an average of 15-20 ms. The risk of QT prolongation is higher in patients with pre-existing cardiac conditions (e.g., hypokalemia, heart failure) or concurrent use of other QT-prolonging drugs [3] 2. Hepatic toxicity: In rare cases, Clarithromycin administration is associated with elevated serum transaminases (ALT, AST) and bilirubin. In a clinical study of 1000 patients treated with Clarithromycin (500 mg twice daily for 14 days), 2.3% of patients had ALT levels >3 times the upper limit of normal (ULN), and 1.1% had AST levels >3 times ULN. The hepatic enzymes returned to normal within 2-4 weeks after drug discontinuation [1] 3. Drug-drug interactions: Due to its inhibition of CYP3A4, Clarithromycin increases the plasma concentrations of co-administered CYP3A4 substrates. For example, concurrent use of Clarithromycin (500 mg twice daily) and cyclosporine (5 mg/kg daily) increases the cyclosporine AUC by 2.5-fold, increasing the risk of nephrotoxicity. Similarly, co-administration with warfarin increases the international normalized ratio (INR) by 1.5-2.0-fold, increasing the risk of bleeding [1,2] 4. Gastrointestinal toxicity: The most common adverse effects of Clarithromycin are gastrointestinal symptoms, including nausea (8%-12%), diarrhea (6%-10%), abdominal pain (4%-6%), and vomiting (2%-4%). These symptoms are usually mild to moderate and resolve with continued treatment or drug discontinuation [1] 5. Plasma protein binding-related toxicity: The concentration-dependent plasma protein binding of Clarithromycin may lead to increased free drug concentrations at high doses (>1000 mg daily), potentially increasing the risk of adverse effects (e.g., headache, dizziness) [1] . |
| 参考文献 |
|
| 其他信息 |
Clarithromycin can cause developmental toxicity according to state or federal government labeling requirements.
Clarithromycin is the 6-O-methyl ether of erythromycin A, clarithromycin is a macrolide antibiotic used in the treatment of respiratory-tract, skin and soft-tissue infections. It is also used to eradicate Helicobacter pylori in the treatment of peptic ulcer disease. It prevents bacteria from growing by interfering with their protein synthesis. It has a role as an antibacterial drug, a protein synthesis inhibitor, an environmental contaminant and a xenobiotic. Clarithromycin is an antibacterial prescription medicine approved by the U.S. Food and Drug Administration (FDA) to treat certain bacterial infections, such as community-acquired pneumonia, throat infections (pharyngitis), acute sinus infections, and others. Clarithromycin is also FDA-approved to both prevent and treat Mycobacterium Avium complex (MAC) infection, another type of bacterial infection. Community-acquired pneumonia, a bacterial respiratory disease, and disseminated MAC infection can be opportunistic infections (OIs) of HIV. An OI is an infection that occurs more frequently or is more severe in people with weakened immune systems—such as people with HIV—than in people with healthy immune systems. Clarithromycin, a semisynthetic macrolide antibiotic derived from erythromycin, inhibits bacterial protein synthesis by binding to the bacterial 50S ribosomal subunit. Binding inhibits peptidyl transferase activity and interferes with amino acid translocation during the translation and protein assembly process. Clarithromycin may be bacteriostatic or bactericidal depending on the organism and drug concentration. Clarithromycin is a Macrolide Antimicrobial. The mechanism of action of clarithromycin is as a Cytochrome P450 3A4 Inhibitor, and Cytochrome P450 3A Inhibitor, and P-Glycoprotein Inhibitor. Clarithromycin is a semisynthetic macrolide antibiotic used for a wide variety of mild-to-moderate bacterial infections. Clarithromycin has been linked to rare instances of acute liver injury that can be severe and even fatal. Clarithromycin is a semisynthetic 14-membered ring macrolide antibiotic. Clarithromycin binds to the 50S ribosomal subunit and inhibits RNA-dependent protein synthesis in susceptible organisms. Clarithromycin has been shown to eradicate gastric MALT (mucosa-associated lymphoid tissue) lymphomas, presumably due to the eradication of tumorigenic Helicobacter pylori infection. This agent also acts as a biological response modulator, possibly inhibiting angiogenesis and tumor growth through alterations in growth factor expression. (NCI04) A semisynthetic macrolide antibiotic derived from ERYTHROMYCIN that is active against a variety of microorganisms. It can inhibit protein synthesis in bacteria by reversibly binding to the 50S ribosomal subunits. This inhibits the translocation of aminoacyl transfer-RNA and prevents peptide chain elongation. See also: Clarithromycin lactobionate (is active moiety of); Amoxicillin; clarithromycin; lansoprazole (component of); Amoxicillin; clarithromycin; vonoprazan fumarate (component of) ... View More ... Drug Indication An alternative medication for the treatment of acute otitis media caused by H. influenzae, M. catarrhalis, or S. pneumoniae in patients with a history of type I penicillin hypersensitivity. Also for the treatment of pharyngitis and tonsillitis caused by susceptible Streptococcus pyogenes, as well as respiratory tract infections including acute maxillary sinusitis, acute bacterial exacerbations of chronic bronchitis, mild to moderate community-acquired pneuomia, Legionnaires' disease, and pertussis. Other indications include treatment of uncomplicated skin or skin structure infections, helicobacter pylori infection, duodenal ulcer disease, bartonella infections, early Lyme disease, and encephalitis caused by Toxoplasma gondii (in HIV infected patients in conjunction with pyrimethamine). Clarithromycin may also decrease the incidence of cryptosporidiosis, prevent the occurence of α-hemolytic (viridans group) streptococcal endocarditis, as well as serve as a primary prevention for Mycobacterium avium complex (MAC) bacteremia or disseminated infections (in adults, adolescents, and children with advanced HIV infection). Clarithromycin is indicated in combination with [vonoprazan] and [amoxicillin] as co-packaged triple therapy to treat _Helicobacter pylori_ (_H. pylori_) infection in adults. FDA Label Treatment of Helicobacter spp. infections Treatment of Helicobacter spp. infections Mechanism of Action Clarithromycin is first metabolized to 14-OH clarithromycin, which is active and works synergistically with its parent compound. Like other macrolides, it then penetrates bacteria cell wall and reversibly binds to domain V of the 23S ribosomal RNA of the 50S subunit of the bacterial ribosome, blocking translocation of aminoacyl transfer-RNA and polypeptide synthesis. Clarithromycin also inhibits the hepatic microsomal CYP3A4 isoenzyme and P-glycoprotein, an energy-dependent drug efflux pump. Clarithromycin usually is bacteriostatic, although it may be bactericidal in high concentrations or against highly susceptible organisms. Bactericidal activity has been observed against Streptococcus pyogenes, S. pneumoniae, Haemophilus influenzae, and Chlamydia trachomatis. Clarithromycin inhibits protein synthesis in susceptible organisms by penetrating the cell wall and binding to 50S ribosomal subunits, thereby inhibiting translocation of aminoacyl transfer-RNA and inhibiting polypeptide synthesis. The site of action of clarithromycin appears to be the same as that of erythromycin, clindamycin, lincomycin, and chloramphenicol. 1. Classification and mechanism of action: Clarithromycin is a semi-synthetic macrolide antibiotic derived from erythromycin. Its antimicrobial mechanism involves binding to the 50S subunit of the bacterial ribosome, which interferes with the translocation step of peptide chain elongation, thereby inhibiting bacterial protein synthesis. This mechanism is bacteriostatic, but it can be bactericidal at high concentrations against susceptible strains [1] 2. Therapeutic indications: Clarithromycin is approved for the treatment of various bacterial infections, including: (1) Upper respiratory tract infections (sinusitis, pharyngitis, tonsillitis) caused by Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis; (2) Lower respiratory tract infections (community-acquired pneumonia, acute exacerbations of chronic bronchitis) caused by Streptococcus pneumoniae, Haemophilus influenzae, Legionella pneumophila, or Mycoplasma pneumoniae; (3) Skin and soft tissue infections caused by Staphylococcus aureus or Streptococcus pyogenes; (4) Prophylaxis and treatment of Mycobacterium avium complex (MAC) infection in immunocompromised patients (e.g., HIV-positive individuals) [1,5] 3. Resistance mechanisms: Bacterial resistance to Clarithromycin primarily occurs through three mechanisms: (1) Methylation of the 23S rRNA in the 50S ribosomal subunit (encoded by erm genes), which reduces drug binding affinity; (2) Efflux pump overexpression (encoded by mef or msr genes), which increases drug extrusion from bacterial cells; (3) Mutations in the 23S rRNA gene, which alter the drug binding site [1] 4. Role in colorectal cancer: Clarithromycin inhibits autophagy in colorectal cancer cells by disrupting the interaction between the HERG1 potassium channel and PI3K, leading to downregulation of the PI3K/Akt/mTOR signaling pathway. This autophagy inhibition enhances the sensitivity of colorectal cancer cells to chemotherapy drugs (e.g., 5-fluorouracil) in preclinical models, suggesting potential use as an adjuvant therapy [4] 5. Advantages over erythromycin: Compared to its parent drug erythromycin, Clarithromycin has several advantages: (1) Better oral bioavailability (50% vs. 35%); (2) Longer half-life (3-4 hours vs. 1-2 hours), allowing twice-daily dosing; (3) Reduced gastrointestinal toxicity (due to decreased affinity for motilin receptors); (4) Enhanced activity against atypical pathogens (Mycoplasma, Chlamydia, Legionella) and MAC [1] . |
| 分子式 |
C38H69NO13
|
|---|---|
| 分子量 |
747.95
|
| 精确质量 |
747.476
|
| 元素分析 |
C, 61.02; H, 9.30; N, 1.87; O, 27.81
|
| CAS号 |
81103-11-9
|
| 相关CAS号 |
Clarithromycin-13C,d3;Clarithromycin-d3;959119-22-3
|
| PubChem CID |
84029
|
| 外观&性状 |
Colorless needles from chloroform + diisopropyl ether (1:2) ... Also reported as crystals from ethanol
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
805.5±65.0 °C at 760 mmHg
|
| 熔点 |
217-220ºC
|
| 闪点 |
440.9±34.3 °C
|
| 蒸汽压 |
0.0±6.5 mmHg at 25°C
|
| 折射率 |
1.526
|
| LogP |
3.16
|
| tPSA |
182.91
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
14
|
| 可旋转键数目(RBC) |
8
|
| 重原子数目 |
52
|
| 分子复杂度/Complexity |
1190
|
| 定义原子立体中心数目 |
18
|
| SMILES |
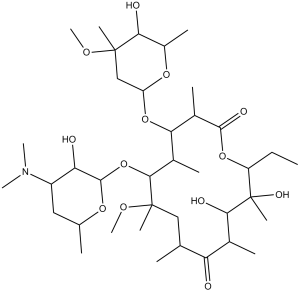
O([C@@H]1O[C@H](C)C[C@H](N(C)C)[C@H]1O)[C@@H]1[C@@H](C)[C@H](O[C@@H]2O[C@@H](C)[C@H](O)[C@](C)(OC)C2)[C@@H](C)C(=O)O[C@H](CC)[C@](O)(C)[C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@@]1(C)OC
|
| InChi Key |
AGOYDEPGAOXOCK-KCBOHYOISA-N
|
| InChi Code |
InChI=1S/C38H69NO13/c1-15-26-38(10,45)31(42)21(4)28(40)19(2)17-37(9,47-14)33(52-35-29(41)25(39(11)12)16-20(3)48-35)22(5)30(23(6)34(44)50-26)51-27-18-36(8,46-13)32(43)24(7)49-27/h19-27,29-33,35,41-43,45H,15-18H2,1-14H3/t19-,20-,21+,22+,23-,24+,25+,26-,27+,29-,30+,31-,32+,33-,35+,36-,37-,38-/m1/s1
|
| 化学名 |
(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-6-(((2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-14-ethyl-12,13-dihydroxy-4-(((2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-7-methoxy-3,5,7,9,11,13-hexamethyloxacyclotetradecane-2,10-dione
|
| 别名 |
Abbott56268; A56268; A-56268; A 56268; A56268; Abbott 56268; A 56268; Clarithromycin; Abbott-56268; A-56268; brand name Biaxin.clarithromycin; 81103-11-9; Biaxin; 6-O-Methylerythromycin; Klaricid; Clarithromycine; Clathromycin; Macladin
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (3.34 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (3.34 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (3.34 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3370 mL | 6.6849 mL | 13.3699 mL | |
| 5 mM | 0.2674 mL | 1.3370 mL | 2.6740 mL | |
| 10 mM | 0.1337 mL | 0.6685 mL | 1.3370 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02790450 | Completed | Drug: Benzbromarone | Idiopathic Pulmonary Arterial Hypertension |
Medical University of Graz | October 2015 | Phase 2 |
| NCT02338323 | Completed | Drug: Febuxostat Drug: Benzbromarone |
Chronic Kidney Disease Hyperuricemia |
Shanghai 10th People's Hospital | January 2015 | Not Applicable |
| NCT03100318 | Completed | Drug: FYU-981 Drug: Benzbromarone |
Hyperuricemia With or Without Gout | Fuji Yakuhin Co., Ltd. | April 1, 2017 | Phase 3 |
| NCT05504083 | Recruiting | Drug: D-0120 Drug: Benzbromarone |
Hyperuricemia | InventisBio Co., Ltd | September 28, 2022 | Phase 2 |
|
|---|
|
|