| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
5-HT2A Receptor ( Ki = 4 nM ); 5-HT6 Receptor; 5-HT7 Receptor; mAChR1 ( Ki = 9.5 nM ); mAChR4 ( Ki = 11 nM ); α2-adrenergic receptor ( Ki = 51 nM ); D2 Receptor ( Ki = 75 nM )
Clozapine (HF 1854) exhibits high affinity for multiple neurotransmitter receptors: - Dopamine D2 receptor: Ki = 12 nM (rat striatal membranes, [³H]-spiperone as radioligand) [2] - Serotonin 5-HT2A receptor: Ki = 6 nM (rat frontal cortex membranes, [³H]-ketanserin as radioligand) [2] - Serotonin 5-HT6 receptor: Ki = 25 nM (human recombinant 5-HT6, [³H]-lysergic acid diethylamide as radioligand) [6] - Clozapine (HF 1854) also binds to α1-adrenergic receptor (Ki = 40 nM, rat brain membranes, [³H]-prazosin) and histamine H1 receptor (Ki = 18 nM, guinea pig brain membranes, [³H]-pyrilamine) [6] |
|---|---|
| 体外研究 (In Vitro) |
氯氮平(10、20 mg/kg)显着增加伏隔核、内侧前额皮质和外侧隔核中 Fos 阳性神经元的数量。氯氮平在大鼠纹状体中诱导 zif268 但不诱导 c-fos mRNA,而氟哌啶醇在尾壳核中诱导 c-Fos 样免疫反应性。与 D2 受体相比,氯氮平对 D4 受体更具选择性。氯氮平是一种混合但较弱的 D1/D2 拮抗剂。 Clozapine 以浓度依赖性方式显着促进 (300-400%) NMDA 诱发反应,EC50 为 14 nM。氯氮平(而不是氟哌啶醇)会产生兴奋性突触后电位 (EPSP) 的爆发,而这种电位会被谷氨酸受体拮抗剂阻断,这表明这些 EPSP 是兴奋性氨基酸释放增加的结果。 Clozapine 是毒蕈碱 M4 受体的完全激动剂 (EC50 = 11 nM),可抑制毛喉素刺激的 cAMP 积累。氯氮平可有效拮抗激动剂诱导的其他四种毒蕈碱受体亚型的反应。
大鼠海马切片谷氨酸释放抑制:大鼠海马切片与氯氮平(1-100 μM)孵育30分钟,呈剂量依赖性抑制K⁺诱导的谷氨酸释放。10 μM时,谷氨酸释放量较溶媒对照组降低45%(孵育液中谷氨酸通过HPLC检测)[1] - 人神经母细胞瘤SH-SY5Y细胞多巴胺摄取调节:氯氮平(0.1-10 μM)处理SH-SY5Y细胞24小时,降低[³H]-多巴胺摄取。5 μM时,摄取量抑制32%,且无显著细胞毒性(台盼蓝染色,活力>90%)[4] - 受体介导的钙动员:在转染人5-HT2A受体的HEK293细胞中,氯氮平(1-50 nM)抑制5-HT(100 nM)诱导的细胞内钙升高。10 nM时,钙响应降低68%(荧光钙指示剂实验)[6] |
| 体内研究 (In Vivo) |
非典型抗精神病药物氯氮平表现出“倒U”形剂量反应曲线,在低剂量下逆转了阿扑吗啡诱导的PPI损失,但在高剂量下没有逆转。高剂量SCH 23390和氯氮平均能降低PPI,与阿扑吗啡治疗无关。阿扑吗啡对基线惊吓幅度的影响也被这些药物不同程度地改变:阿扑吗啡增强了斯皮哌酮和雷氯匹啶预处理动物的惊吓幅度,但阿扑吗啡降低了SCH 23390或高剂量氯氮平预处理的动物的惊吓幅度。研究表明,精神分裂症患者的脉前抑制明显受损。由于我们目前的研究结果表明,D2多巴胺受体的激活是大鼠PPI损失的原因,因此D2多巴胺受体过度活动也可能是精神分裂症PPI缺陷的底物。[6]
本实验通过比较氯氮平和氟哌啶醇对内侧前额叶皮层、伏隔核、纹状体和外侧隔c-fos表达的影响,研究了氯氮平和氟哌利多作用的一些潜在神经解剖学差异。还研究了选择性多巴胺受体拮抗剂SCH 23390(D1)和雷氯匹定(D2)的作用。氟哌啶醇(0.5,1mg/kg)和雷氯必利(1,2mg/kg)显著增加了纹状体和伏隔核中含Fos神经元的数量。SCH 23390(0.5,1mg/kg)减少了伏隔核和纹状体中Fos阳性神经元的数量,对其他区域没有影响。氟哌啶醇和雷氯必利均未增加内侧前额叶皮层Fos阳性神经元的数量。氟哌啶醇(而非雷氯匹林)使外侧隔核中c-fos的表达略有增加。氯氮平(10、20mg/kg)对纹状体无影响;然而,它显著增加了伏隔核、内侧前额叶皮层和外侧隔核中Fos阳性神经元的数量。用6-羟基多巴胺破坏中脑多巴胺能神经元,可以消除氟哌啶醇和雷氯必利在伏隔核和纹状体中Fos表达的增加,也可以阻断氯氮平诱导的伏隔核增加。然而,氯氮平和氟哌啶醇对外侧隔核c-fos表达的诱导作用以及氯氮平对内侧前额叶皮层c-fos表达的促进作用不受6-羟基多巴胺损伤的影响。这些结果表明,氯氮平的独特治疗特征可能与它未能在纹状体中诱导Fos以及在外侧隔膜和内侧前额叶皮层中的特异性作用有关。氯氮平在这些后一区域的作用似乎不是由多巴胺能机制介导的。[1] 氯氮平在大鼠中表现出倒 U 形剂量反应曲线,低剂量时可逆转阿朴吗啡引起的前脉冲抑制 (PPI) 丧失,但高剂量时则不能逆转。氯氮平可降低大鼠的 PPI,与阿扑吗啡治疗无关。 小鼠阿扑吗啡诱导刻板行为模型:雄性ICR小鼠(20-25 g)腹腔注射阿扑吗啡(2 mg/kg)诱导刻板行为(嗅探、舔舐)。阿扑吗啡处理前30分钟腹腔注射氯氮平(5 mg/kg),刻板行为评分降低65%(行为评分:0-4分制)[3] - 大鼠苯环利定(PCP)诱导过度活动模型:雌性SD大鼠(250-300 g)皮下注射PCP(5 mg/kg)诱导过度活动。PCP处理前1小时口服氯氮平(10 mg/kg),活动距离降低52%(旷场实验,记录30分钟移动距离)[3] - 猫急性神经行为实验:雄性家猫(2-3 kg)静脉注射氯氮平(0.5 mg/kg),呈剂量依赖性镇静(梳理行为减少),且不诱导僵住症(横杆实验:维持姿势>60秒)[5] |
| 酶活实验 |
在中国仓鼠卵巢细胞中表达的人毒蕈碱M1-M5受体的功能测定中研究了氯氮平。氯氮平是毒蕈碱M4受体的完全激动剂(EC50=11nM),可抑制毛喉素刺激的cAMP积累。相比之下,氯氮平能有效拮抗激动剂诱导的其他四种毒蕈碱受体亚型的反应。选择性刺激M4受体可能部分解释氯氮平临床上观察到的高唾液分泌。此外,氯氮平独特的总体毒蕈碱特征可能有助于其非典型抗精神病疗效[4]。
多巴胺D2受体结合实验:200 μL反应体系包含50 μg大鼠纹状体膜蛋白、0.5 nM [³H]-螺哌隆(放射性配体)及氯氮平(0.1-100 nM)。25°C孵育60分钟后,通过玻璃纤维滤膜过滤分离结合态与游离态配体。滤膜用冷50 mM Tris-HCl(pH 7.4)洗涤3次,液体闪烁计数仪检测放射性。非特异性结合通过加入1 μM氟哌啶醇确定,采用Cheng-Prusoff方程计算Ki[2] - 5-HT2A受体结合实验:150 μL反应体系包含40 μg大鼠额叶皮层膜蛋白、0.3 nM [³H]-酮色林及氯氮平(0.05-50 nM)。37°C孵育45分钟后,通过预浸泡玻璃纤维滤膜过滤。滤膜用冷10 mM磷酸钠缓冲液(pH 7.4)洗涤,定量放射性。非特异性结合通过加入10 μM米安色林确定,从竞争结合曲线推导Ki[6] |
| 细胞实验 |
采用细胞内记录和单电极电压钳技术,在大鼠脑切片内侧前额叶皮层锥体细胞中检测并比较了抗精神病药物氟哌啶醇和氯氮平对N-甲基-D-天冬氨酸(NMDA)和非NMDA受体介导的神经传递的影响。氟哌啶醇或氯氮平的浴给药以浓度依赖的方式显著促进了NMDA诱发的反应(300-400%)。氟哌啶醇和氯氮平的EC50值分别为38和14 nM。当浓度大于或等于100 nM时,氯氮平(而不是氟哌啶醇)会产生兴奋性突触后电位(EPSPs)的爆发,这些电位被谷氨酸受体拮抗剂阻断,这表明这些EPSPs是兴奋性氨基酸释放增加的结果。氟哌啶醇(而非氯氮平)对α-氨基-3-羟基-5-甲基-4-异恶唑丙酸诱导的电流产生浓度依赖性抑制,EC50值为37 nM。氟哌啶醇显著降低了电刺激小镊子诱发的EPSP的振幅,而氯氮平则增加了这些EPSP的幅度。电流-电压关系的研究表明,氯氮平优先增强NMDA受体介导的传输,而氟哌啶醇抑制非NMDA受体中介的反应,这可能掩盖了它对NMDA受体诱导的EPSPs的增强作用[3]。
大鼠海马切片谷氨酸释放实验:从雄性Wistar大鼠(150-200 g)分离海马,振动切片机切成300 μm切片。切片在Krebs-Ringer缓冲液(37°C,95% O₂/5% CO₂)中预孵育60分钟,加入氯氮平(1-100 μM)继续孵育30分钟。加入50 mM KCl诱导谷氨酸释放,15分钟后收集缓冲液,反相HPLC紫外检测谷氨酸浓度。结果以切片蛋白浓度(BCA法)归一化[1] - SH-SY5Y细胞多巴胺摄取实验:SH-SY5Y细胞以2×10⁵细胞/孔接种于24孔板,含10% FBS的DMEM/F12培养基培养48小时。加入氯氮平(0.1-10 μM)孵育24小时,更换为含0.1 nM [³H]-多巴胺的缓冲液,37°C孵育10分钟。冷缓冲液洗涤细胞3次,0.1 M NaOH裂解细胞,检测放射性。非特异性摄取通过加入10 μM诺米芬辛确定[4] |
| 动物实验 |
25 mg/kg
Mice: Mice are treated chronically (21 days) with 25 mg/kg/day clozapine. Experiments are conducted 1, 7, 14, and 21 days after the last clozapine administration. [3H]Ketanserin binding and 5-HT2A mRNA expression are determined in mouse somatosensory cortex. Head-twitch behavior, expression of c-fos, which is induced by all 5-HT2A agonists, and expression of egr-1 and egr-2, which are LSD-like specific, are assayed Mouse Apomorphine Stereotypy Model: Male ICR mice (6-8 weeks old, 20-25 g) were housed under SPF conditions (22±2°C, 12-hour light/dark cycle). Mice were randomized into 3 groups (n=8/group): 1. Vehicle control: Intraperitoneal injection of 0.9% saline (10 mL/kg); 2. Apomorphine-only: Intraperitoneal injection of apomorphine (2 mg/kg, dissolved in saline); 3. Clozapine+Apomorphine: Intraperitoneal injection of Clozapine (5 mg/kg, dissolved in 0.1% DMSO+saline) 30 minutes before apomorphine. Stereotyped behavior was scored every 10 minutes for 60 minutes (0: no stereotypy; 4: continuous stereotypy). Total scores were calculated for each group [3] - Rat PCP Hyperlocomotion Model: Female Sprague-Dawley rats (8-10 weeks old, 250-300 g) were randomized into 3 groups (n=6/group): 1. Vehicle control: Oral gavage of 0.5% carboxymethylcellulose sodium (CMC-Na, 10 mL/kg); 2. PCP-only: Subcutaneous injection of PCP (5 mg/kg, dissolved in saline); 3. Clozapine+PCP: Oral gavage of Clozapine (10 mg/kg, dissolved in 0.5% CMC-Na) 1 hour before PCP. Rats were placed in an open-field arena (40×40×30 cm) 30 minutes after PCP injection, and locomotor activity (distance traveled) was recorded for 30 minutes via video tracking [3] - Cat Intravenous Sedation Assay: Male domestic cats (2-3 kg) were anesthetized with isoflurane for catheterization of the jugular vein. After recovery (24 hours), cats were injected intravenously with Clozapine (0.1-1 mg/kg, dissolved in sterile saline). Behavioral observations (sedation, grooming, catalepsy) were performed every 15 minutes for 2 hours. Catalepsy was assessed via the bar test (cat placed on a 1 cm diameter bar, time to fall recorded) [5] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
In humans, clozapine tablets (25 mg and 100 mg) are equally bioavailable relative to a CLOZARIL solution. Following oral administration of clozapine 100 mg twice daily, the average steady-state peak plasma concentration was 319 ng/mL (range: 102 to 771 ng/mL), occurring at the average of 2.5 hours (range: 1 to 6 hours) after dosing. The average minimum concentration at steady state was 122 ng/mL (range: 41 to 343 ng/mL), after 100 mg twice daily dosing. Approximately 50% of the administered dose is excreted in the urine and 30% in the feces. The median volume of distribution of clozapine was calculated to be 508 L (272–1290 L). The median clearance of clozapine is calculated to be 30.3 L/h (14.4–45.2 L/h). Clozapine is almost completely metabolized prior to excretion and only trace amounts of unchanged drug are detected in the urine and feces. Approximately 50% of the administered dose is excreted in the urine and 30% in the feces. The demethylated, hydroxylated and N-oxide derivatives are components in both urine and feces. Pharmacological testing has shown the desmethyl metabolite to have only limited activity, while the hydroxylated and N-oxide derivatives were inactive. In man, clozapine tablets (25 mg and 100 mg) are equally bioavailable relative to a clozapine solution. Following a dosage of 100 mg b.i.d., the average steady-state peak plasma concentration was 319 ng/mL (range: 102 to 771 ng/mL), occurring at the average of 2.5 hours (range: 1 to 6 hours) after dosing. The average minimum concentration at steady-state was 122 ng/mL (range: 41 to 343 ng/mL), after 100 mg b.i.d. dosing. Food does not appear to affect the systemic bioavailability of clozapine. Thus, clozapine may be administered with or without food. Clozapine is approximately 97% bound to serum proteins. Clozapine is rapidly absorbed after both single and repeated oral doses, with steady-state concentrations attained within eight to ten days after beginning therapy. Metabolism / Metabolites Clozapine is almost completely metabolized prior to excretion, and only trace amounts of unchanged drug are detected in the urine and feces. Clozapine is a substrate for many cytochrome P450 isozymes, in particular CYP1A2, CYP2D6, and CYP3A4.The unmethylated, hydroxylated, and N-oxide derivatives are components in both urine and feces. Pharmacological testing has shown the desmethyl metabolite (norclozapine) to have only limited activity, while the hydroxylated and N-oxide derivatives were inactive. Manic and schizophrenic patients were given neuroleptic clozapine at 300-500 mg daily and metabolites of clozapine were isolated from urine and analyzed by gas chromatography-mass spectrometry. Clozapine was converted into 2 metabolites by replacement of chlorine atom by a hydroxyl or methylsulfide group. Further metabolites were the N-demethyl deriv of 1st two metabolites. A metabolite with an oxidized piperazine ring was also found, and possibility of a metabolite with an oxidized sulfur is suggested. /Clozapine/ is metabolized to N-oxideclozapine and N-desmethylclozapine, which have less pharmacological activity than the parent compound and are excreted in the urine and, to a lesser extent, in the feces. Clozapine has known human metabolites that include Clozapine N-glucuronide, Clozapine-N-oxide, and N-Desmethylclozapine. Biological Half-Life The mean elimination half-life of clozapine after a single 75 mg dose was 8 hours (range: 4 to 12 hours), compared to a mean elimination half-life of 12 hours (range: 4 to 66 hours), after achieving a steady state with 100 mg twice daily dosing. A comparison of single-dose and multiple-dose administration of clozapine demonstrated that the elimination half-life increased significantly after multiple dosing relative to that after single-dose administration, suggesting the possibility of concentration-dependent pharmacokinetics. The mean elimination half-life of clozapine after a single 75 mg dose was 8 hours (range: 4 to 12 hours), compared to a mean elimination half-life, after achieving steady-state with 100 mg b.i.d. dosing, of 12 hours (range: 4 to 66 hours). Oral bioavailability: In male Sprague-Dawley rats, oral administration of Clozapine (20 mg/kg) showed an oral bioavailability of 35% compared to intravenous administration (10 mg/kg) [4] - Plasma pharmacokinetics: Rats intravenously injected with Clozapine (10 mg/kg) had a Cmax of 1.8 μg/mL, Tmax of 5 minutes, and elimination half-life (t1/2) of 2.2 hours. Oral administration (20 mg/kg) resulted in Cmax of 0.6 μg/mL and Tmax of 1.5 hours [4] - Tissue distribution: In mice, 1 hour after intraperitoneal injection of Clozapine (10 mg/kg), the brain/plasma concentration ratio was 2.8, with highest accumulation in the frontal cortex and striatum (HPLC detection of clozapine in tissue homogenates) [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Clozapine has been shown to be an effective, relatively rapid-acting, broad-spectrum antipsychotic agent in both uncontrolled and controlled studies of patients with schizophrenia.Clozapine has been used in a limited number of patients with advanced, idiopathic parkinsonian syndrome for the management of dopaminomimetic psychosis associated with antiparkinsonian drug therapy, but adverse effects such as sedation, confusion, and increased parkinsonian manifestations may limit the benefit of clozapine therapy in these patients. Clozapine is used to reduce the risk of recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder who are judged to be at chronic risk for such behavior, based on history and recent clinical state. Although the safety and efficacy of clozapine in children and adolescents younger than 16 years of age have not been established, the drug has been successfully used for the management of childhood-onset schizophrenia in a limited number of treatment-resistant children and adolescents. Clozapine is used for the symptomatic management of schizophrenia in severely ill patients whose disease fails to respond adequately to other antipsychotic therapy. HUMAN EXPOSURE AND TOXICITY: The most frequent adverse effects of clozapine involve the central and autonomic nervous systems (e.g., drowsiness or sedation, hypersalivation) and the cardiovascular system (e.g., tachycardia, hypotension). While the frequency and severity of some adverse effects (e.g., extrapyramidal reactions, tardive dyskinesia) appear to be less with clozapine than with other antipsychotic agents, other potentially serious adverse effects (e.g., agranulocytosis, seizures) may occur more frequently with clozapine therapy, and the potential risks and benefits should be evaluated carefully whenever therapy with the drug is considered. Although it has been suggested that a local genetic or environmental factor or factors may have been involved in the Finnish cases, the existence of such a factor has not been documented. During a 2 month period in Finland there were 18 reports of severe blood disorders (9 fatal) associated with clozapine. Agranulocytosis accounted for 8 of the deaths and leukemia probably for the ninth. Experience in 22 other countries outside Finland where clozapine had been marketed indicated an incidence of agranulocytosis of 0.3 per 1000 compared with an incidence almost 20 times as high in Finland and with 0.1 to 0.8 per 1000 for other tricyclic neuroleptics. patients who received flexible dosages of clozapine (mean dosage: 274.2 mg daily) for approximately 2 years had a 26% reduction in their risk for suicide attempts or hospitalization to prevent suicide compared with those who received flexible dosages of olanzapine (mean dosage: 16.6 mg daily); the treatment-resistant status of patients was not predictive of response to clozapine or olanzapine. ANIMAL STUDIES: Repeated oral administration to rats (6 months) and to dogs (3 months) decreased wt gain with doses of 20 mg/kg or more in rats and of 10 mg/kg or more in dogs. Hepatic hypertrophy, which was not strictly dose-dependent, was not associated with either histological changes or changes in blood chemistry and was completely reversible after discontinuation of treatment. No toxic signs were observed in rats or in dogs. Clozapine in daily oral doses of 20 or 40 mg/kg to rats and rabbits had no teratogenic effects and no influence on the fertility of male and female rats. At 40 mg/kg, however, clozapine inhibited growth of suckling young of treated mothers. Fertility of F1 treated mothers was normal and development of F2 showed no abnormalities. Clozapine hydrochloride inhibited conditioned avoidance behavior in rats, also inhibited writhing syndrome induced by phenylbenzoquinone in mice, and decreased body temp. Clozapine hydrochloride antagonized tremor and lacrimation induced by oxotremorine in mice, decreased the acute toxicity of physostigmine and 5-hydroxyindol acetate level in brain. Non-Human Toxicity Values LD50 Rat iv 41.6 mg/kg LD50 Rat sc 240 mg/kg LD50 Rat im 210 mg/kg LD50 Rat oral 251 mg/kg Acute in vivo toxicity: Male ICR mice intraperitoneally injected with Clozapine had an LD50 of 120 mg/kg. Mice treated with doses >80 mg/kg showed symptoms of respiratory depression and ataxia, which were fatal within 24 hours [5] - Subacute toxicity: Rats orally administered Clozapine (10 mg/kg/day) for 14 days showed no significant changes in serum ALT, AST, BUN, or creatinine levels. Histopathological examination of liver and kidney revealed no tissue damage [4] - Plasma protein binding: In human plasma, Clozapine showed >95% protein binding (ultrafiltration method, plasma concentration 0.1-10 μg/mL) [2] |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Antipsychotic Agents; GABA Antagonists; Serotonin Antagonists Clozapine tablets are indicated for the management of severely ill schizophrenic patients who fail to respond adequately to standard drug treatment for schizophrenia. Because of the significant risk of agranulocytosis and seizure associated with its use, clozapine tablets should be used only in patients who have failed to respond adequately to treatment with appropriate courses of standard drug treatments for schizophrenia, either because of insufficient effectiveness or the inability to achieve an effective dose due to intolerable adverse effects from those drugs. Clozapine has been used in a limited number of patients with advanced, idiopathic parkinsonian syndrome for the management of dopaminomimetic psychosis associated with antiparkinsonian drug therapy, but adverse effects such as sedation, confusion, and increased parkinsonian manifestations may limit the benefit of clozapine therapy in these patients. Attempts to relieve antiparkinsonian drug-induced delusions, paranoia, and hallucinations by reduction of antiparkinsonian drug dosage or administration of typical antipsychotic agents often aggravate parkinsonian symptoms. Limited data suggest that administration of clozapine in dosages of 6.25-400 mg daily can improve psychotic symptoms within a few days, reportedly without exacerbating parkinsonian manifestations. However, in a controlled study in a limited number of patients receiving clozapine dosages up to 250 mg daily, exacerbation of parkinsonian manifestations and development of delirium occurred frequently despite prevention of antiparkinsonian drug-induced deterioration of psychosis;88 it has been suggested that rapid clozapine dosage escalation may have contributed to the observed negative effect on parkinsonian manifestations and delirium. Clozapine is used to reduce the risk of recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder who are judged to be at chronic risk for such behavior, based on history and recent clinical state. Efficacy of clozapine for this indication has been established in a multicenter, randomized, open-label clinical study (the International Suicide Prevention Trial [InterSePT]) of 2 years' duration comparing clozapine and olanzapine in patients with schizophrenia (62%) or schizoaffective disorder (38%) who were judged to be at risk for recurrent suicidal behavior. For more Therapeutic Uses (Complete) data for CLOZAPINE (8 total), please visit the HSDB record page. Drug Warnings BOXED WARNING: 1. AGRANULOCYTOSIS: Because of a significant risk of agranulocytosis, a potentially life threatening adverse event, clozapine should be reserved for use in (1) the treatment of severely ill patients with schizophrenia who fail to show an acceptable response to adequate courses of standard antipsychotic drug treatment, or (2) for reducing the risk of recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder who are judged to be at risk of re-experiencing suicidal behavior. Patients being treated with clozapine must have a baseline white blood cell (WBC) count and absolute neutrophil count (ANC) before initiation of treatment as well as regular WBC counts and ANCs during treatment and for at least 4 weeks after discontinuation of treatment. Clozapine is available only through a distribution system that ensures monitoring of WBC count and ANC according to the schedule described below prior to delivery of the next supply of medication. BOXED WARNING: 2. SEIZURES: Seizures have been associated with the use of clozapine. Dose appears to be an important predictor of seizure, with a greater likelihood at higher clozapine doses. Caution should be used when administering clozapine to patients having a history of seizures or other predisposing factors. Patients should be advised not to engage in any activity where sudden loss of consciousness could cause serious risk to themselves or others. BOXED WARNING: 3. MYOCARDITIS: Analyses of post-marketing safety databases suggest that clozapine is associated with an increased risk of fatal myocarditis, especially during, but not limited to, the first month of therapy. In patients in whom myocarditis is suspected, clozapine treatment should be promptly discontinued. BOXED WARNING: 4. OTHER ADVERSE CARDIOVASCULAR AND RESPIRATORY EFFECTS: Orthostatic hypotension, with or without syncope, can occur with clozapine treatment. Rarely, collapse can be profound and be accompanied by respiratory and/or cardiac arrest. Orthostatic hypotension is more likely to occur during initial titration in association with rapid dose escalation. In patients who have had even a brief interval off clozapine, i.e., 2 or more days since the last dose, treatment should be started with 12.5 mg once or twice daily. Since collapse, respiratory arrest and cardiac arrest during initial treatment has occurred in patients who were being administered benzodiazepines or other psychotropic drugs, caution is advised when clozapine is initiated in patients taking a benzodiazepine or any other psychotropic drug. For more Drug Warnings (Complete) data for CLOZAPINE (20 total), please visit the HSDB record page. Pharmacodynamics Clozapine is a psychotropic agent belonging to the chemical class of benzisoxazole derivatives that is universally regarded as the treatment of choice for treatment-resistant schizophrenia. Although it is thought to mediate its pharmacological effect through antagonism of the dopamine type 2 (D2) and the serotonin type 2A (5-HT2A) receptors, research have shown that clozapine can act on various types of receptors. Patients should be counseled regarding the risk of hypersensitivity reactions such as agranulocytosis and myocarditis with clozapine use. Clozapine-induced agranulocytosis, which is a reduction in the absolute neutrophil count or white blood cell count, places the patient at an increased risk for infection. Agranulocytosis is most likely to occur in the first 3-6 months of therapy, but it can still occur after years of treatment. The mechanism is thought to be a dose-independent and immune-mediated reaction against neutrophils. Patients are strictly monitored by lab testing (complete blood count with differential) to ensure agranulocytosis is detected and treated if it occurs. Testing is initially completed at one-week intervals but is expanded to two-week intervals at six months, and then four-week intervals at twelve months if lab results have been within an appropriate range. Monitoring parameters may change if there is any break in therapy. In Canada, the patient's lab values are reported to the manufacturer for hematological monitoring, and in the USA, the patient's lab values are reported to the REMS (Risk Evaluation and Mitigation Strategy) program. These programs function to notify the care provider of any significant drop in WBC/neutrophil count, or if there is a drop below a threshold level. Patients who enter the \"Red\" zone (WBC<2x109/L or ANC<1.5x109/L) should normally not be re-challenged. Clozapine-induced myocarditis is a hypersensitivity reaction that usually occurs in the third week of clozapine therapy and about 2% of clozapine patients. Monitor the patient's troponin, CRP, and ECG at baseline, and 28 days into treatment. Follow guidelines for appropriate next steps according to the patient's lab results. If myocarditis occurs, the patient should not be re-challenged with clozapine. Mechanism of action: Clozapine (HF 1854) is an atypical antipsychotic that exerts therapeutic effects by balancing dopamine D2 receptor antagonism (reducing positive symptoms of schizophrenia) and serotonin 5-HT2A receptor antagonism (improving negative symptoms). It also modulates other receptors (α1-adrenergic, H1) to reduce side effects like extrapyramidal symptoms [3,6] - Therapeutic potential: Clozapine is indicated for treatment-resistant schizophrenia (patients unresponsive to typical antipsychotics). In the rat PCP model (a preclinical model of schizophrenia), it showed efficacy at doses of 5-10 mg/kg without inducing catalepsy (a marker of extrapyramidal side effects) [3,5] - Chemical properties: Clozapine (HF 1854) is soluble in DMSO (10 mg/mL) and slightly soluble in saline (0.5 mg/mL). It is stable in aqueous solution at pH 7.4 for 24 hours at 4°C [4] |
| 分子式 |
C18H19CLN4
|
|
|---|---|---|
| 分子量 |
326.82
|
|
| 精确质量 |
326.129
|
|
| 元素分析 |
C, 66.15; H, 5.86; Cl, 10.85; N, 17.14
|
|
| CAS号 |
5786-21-0
|
|
| 相关CAS号 |
Clozapine-d8; 1185053-50-2; Clozapine-d4; 204395-52-8; Clozapine N-oxide; 34233-69-7
|
|
| PubChem CID |
135398737
|
|
| 外观&性状 |
White to light yellow solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
489.2±55.0 °C at 760 mmHg
|
|
| 熔点 |
182-185°C
|
|
| 闪点 |
249.6±31.5 °C
|
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
|
| 折射率 |
1.681
|
|
| LogP |
2.36
|
|
| tPSA |
30.87
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
1
|
|
| 重原子数目 |
23
|
|
| 分子复杂度/Complexity |
446
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
ClC1C([H])=C([H])C2=C(C=1[H])N=C(C1=C([H])C([H])=C([H])C([H])=C1N2[H])N1C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C1([H])[H]
|
|
| InChi Key |
QZUDBNBUXVUHMW-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3
|
|
| 化学名 |
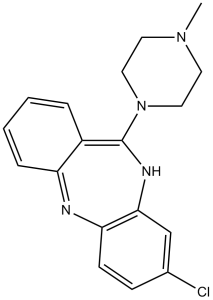
3-chloro-6-(4-methylpiperazin-1-yl)-11H-benzo[b][1,4]benzodiazepine
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.65 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.65 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (7.65 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5%DMSO + Corn oil: 5.0mg/ml (15.30mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0598 mL | 15.2989 mL | 30.5979 mL | |
| 5 mM | 0.6120 mL | 3.0598 mL | 6.1196 mL | |
| 10 mM | 0.3060 mL | 1.5299 mL | 3.0598 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Clozapine for the Prevention of Violence in Schizophrenia: a Randomized Clinical Trial
CTID: NCT05208190
Phase: Phase 4 Status: Recruiting
Date: 2024-04-02