| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Cobicistat is a potent and selective inhibitor of human cytochrome P450 3A (CYP3A) isoforms:
- CYP3A4: IC₅₀ = 0.11 ± 0.03 μM (recombinant enzyme assay) - CYP3A5: IC₅₀ = 0.05 ± 0.01 μM (recombinant enzyme assay) It shows >200-fold selectivity over other CYP isoforms (CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6) with IC₅₀ >25 μM [1]. |
|---|---|
| 体外研究 (In Vitro) |
在抗病毒细胞测试和 HIV-1 蛋白酶检测中,考比司他对 HIV-1 蛋白酶没有活性(IC50>30 μM)。在多周期、为期五天的 MT-2 HIV 感染实验中,考比司他并未减少 HIV 复制(EC50>30 μM)。 Cobicistat 在 MT-2 细胞测试中显示没有细胞毒性,CC50 值大于 80 μM [1]。利托那韦和考比司他通过相同的机制抑制 CYP3A。这意味着CYP3A酶的血红素基团可能直接参与其对CYP3A的抑制作用[1]。在测量人类脂肪细胞脂质积累的实验中,利托那韦表现出显着的效果,EC50 为 16 μM。然而,在剂量高达 30 μM 时,考比司他没有效果 [1]。当浓度为 10 μM 时,利托那韦显着影响小鼠脂肪细胞葡萄糖吸收测定。相反,考比司他 (10 μM) 对葡萄糖吸收的影响要小得多 [1]。
Cobicistat 抑制人肝微粒体中咪达唑仑 1'-羟基化反应,IC₅₀ = 0.20 ± 0.08 μM,表明其在生理相关系统中具有强效 CYP3A 抑制活性 [1]。 时间依赖性抑制研究显示 cobicistat 对 CYP3A4 具有基于机制的抑制作用,KI = 0.67 μM,kinact = 0.017 min⁻¹ [1]。 在 Caco-2 细胞单层中,cobicistat 是 P-糖蛋白的底物(外排比 = 1.9),但在浓度 ≤10 μM 时不显著抑制 P-gp 介导的地高辛转运 [1]。 |
| 体内研究 (In Vivo) |
Cobicistat是一种新型的细胞色素P450 3A4(CYP3A4)抑制剂,在高级临床评估中用作抗逆转录病毒药物的药物增强剂。它缺乏显著的抗HIV活性,在酶抑制方面比利托那韦更具选择性。此外,其水溶性可能适合与其他药物共配制。肾脏不良反应和相当大的药物相互作用潜力限制了其临床实用性,使用时需要谨慎。最近批准了一种固定剂量的联合产品,该产品除依维来韦、替诺福韦和恩曲他滨外,还含有cobicistat,并提供一天一次的抗逆转录病毒方案[2]。
|
| 酶活实验 |
重组 CYP 抑制实验:将重组人 CYP 亚型与 cobicistat(0.001-30 μM)及 CYP 特异性荧光底物在磷酸盐缓冲液中孵育。NADPH 启动反应后,通过荧光测定计算酶活性和 IC₅₀ 值 [1]。
人肝微粒体实验:人肝微粒体与 cobicistat(0.003-10 μM)和 NADPH 预孵育。加入咪达唑仑作为底物,通过 LC-MS/MS 定量 1'-羟基咪达唑仑生成量以确定 IC₅₀ [1]。 基于机制的抑制实验:人肝微粒体与不同浓度 cobicistat(0.1-10 μM)和 NADPH 预孵育 0-30 分钟。稀释后采用睾酮 6β-羟基化实验测定残余 CYP3A4 活性以确定 KI 和 kinact [1]。 |
| 细胞实验 |
Caco-2 渗透性实验:在转运缓冲液中用 cobicistat(10 μM)孵育 Caco-2 细胞单层。在 0-120 分钟收集顶侧和基底侧样品,通过 LC-MS/MS 分析计算表观渗透性(Papp)和外排比 [1]。
P-gp 抑制实验:Caco-2 细胞与地高辛(P-gp 底物)和 cobicistat(0.1-30 μM)共孵育。测定地高辛转运以评估 P-gp 抑制潜力 [1]。 |
| 动物实验 |
Rat Pharmacokinetic Study: Sprague-Dawley rats received single oral doses of cobicistat (10 mg/kg) suspended in 0.5% methylcellulose/0.2% Tween 80. Serial blood samples were collected over 24 hours for PK analysis [1].
Drug Interaction Study in Rats: Rats were pretreated with oral cobicistat (10 mg/kg) or vehicle. After 30 min, midazolam (2 mg/kg) was administered orally. Blood samples were collected for midazolam PK assessment [1].
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Median peak plasma concentrations were observed at 3.5 hours post-dose. With single dose administration of [14C] cobicistat after multiple dosing of cobicistat for six days, the mean percent of the administered dose excreted in feces and urine was 86.2% and 8.2%, respectively. Metabolism / Metabolites Cobicistat is metabolized by CYP3A and to a minor extent by CYP2D6 enzymes and does not undergo glucuronidation. Biological Half-Life The terminal plasma half-life of cobicistat is approximately 3 to 4 hours. Absorption: Oral bioavailability in rats is 39% [1]. Distribution: Volume of distribution at steady state (Vss) is 1.2 L/kg in rats [1]. Metabolism: Primarily metabolized by CYP3A4 and CYP2D6, with oxidative metabolism as major clearance pathway [1]. Excretion: Biliary excretion is significant (≤80% of dose in bile-duct cannulated rats) [1]. Human PK Parameters: At 150 mg dose in humans: Cmax ≈ 1.0 μg/mL, AUC0-24 ≈ 8.0 μg·h/mL, t₁/₂ ≈ 3-4 hr [2]. |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
97-98% bound to human plasma proteins. Cobicistat increases serum creatinine by ~0.1-0.2 mg/dL through inhibition of renal tubular secretion without affecting actual glomerular filtration rate [2]. Clinical trials show common adverse effects: nausea (11-14%), diarrhea (9-12%), and headache (5-8%) [2]. No significant hepatotoxicity observed at therapeutic doses [2]. |
| 参考文献 | |
| 其他信息 |
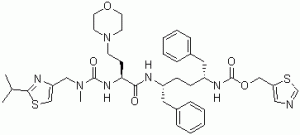
Cobicistat is a monocarboxylic acid amide obtained by formal condensation of the carboxy group of (2S)-2-({[(2-isopropyl-1,3-thiazol-4-yl)methyl](methyl)carbamoyl}amino)-4-(morpholin-4-yl)butanoic acid with the amino group of 1,3-thiazol-5-ylmethyl [(2R,5R)-5-amino-1,6-diphenylhexan-2-yl]carbamate. Acts as a pharmacoenhancer in treatment of HIV-1 by inhibiting P450 enzymes that metabolise other medications.. It has a role as a P450 inhibitor. It is a member of 1,3-thiazoles, a member of morpholines, a member of ureas, a carbamate ester and a monocarboxylic acid amide.
Cobicistat (brand name: Tybost) is a prescription medicine approved by the U.S. Food and Drug Administration (FDA) for use in adults and children along with the HIV medicines atazanavir (brand name Reyataz) or darunavir (brand name Prezista). Cobicistat is not active against HIV and is only used as a pharmacokinetic enhancer to boost the activity of other HIV medicines. When taken with atazanavir, cobicistat is approved for use in adults and children who weigh at least 77 lb (35 kg). (A fixed-dose combination tablet containing atazanavir and cobicistat [brand name: Evotaz] is also available.) When taken with darunavir, cobicistat is approved for use in adults and children who weigh at least 88 lb (40 kg). (A fixed-dose combination tablet containing darunavir and cobicistat [brand name: Prezcobix] is also available.) Cobicistat, marketed under the name Tybost (formerly GS-9350), indicated for treating infection with human immunodeficiency virus (HIV). Although it does not have any anti-HIV activity, cobicistat acts as a pharmacokinetic enhancer by inhibiting cytochrome P450 3A isoforms (CYP3A) and therefore increases the systemic exposure of coadministered agents that are metabolized by CYP3A enzymes. More specifically, cobicistat is indicated to increase systemic exposure of atazanavir or darunavir (once daily dosing regimen) in combination with other antiretroviral agents in the treatment of HIV-1 infection. Increasing systemic exposure of anti-retrovirals (ARVs) without increasing dosage allows for better treatment outcomes and a decreased side effect profile. Cobicistat is a Cytochrome P450 3A Inhibitor. The mechanism of action of cobicistat is as a Cytochrome P450 3A Inhibitor, and P-Glycoprotein Inhibitor, and Cytochrome P450 2D6 Inhibitor, and Organic Anion Transporting Polypeptide 1B1 Inhibitor, and Organic Anion Transporting Polypeptide 1B3 Inhibitor, and Breast Cancer Resistance Protein Inhibitor, and Multidrug and Toxin Extrusion Transporter 1 Inhibitor. Cobicistat is a cytochrome P450 3A (CYP3A) inhibitor that can be used to enhance the pharmacokinetic profile of certain anti-HIV-1 agents. Upon administration, cobicistat inhibits the liver enzyme CYP3A4 and limits the breakdown of co-administered agents that are metabolized by CYP3A4, and increases the concentration, systemic exposure and efficacy of the co-administered agent. A carbamate and thiazole derivative that functions as a CYTOCHROME P450 CYP3A INHIBITOR to enhance the concentration of ANTI-HIV AGENTS, with which it is used in combination, for the treatment of HIV INFECTIONS. See also: Cobicistat; darunavir (component of); Atazanavir sulfate; cobicistat (component of); Cobicistat; darunavir ethanolate (component of) ... View More ... Drug Indication Cobicistat is a CYP3A inhibitor indicated to increase systemic exposure of atazanavir or darunavir (once daily dosing regimen) in combination with other antiretroviral agents in the treatment of HIV-1 infection. It is not interchangeable with ritonavir to increase systemic exposure of darunavir 600 mg twice daily, fosamprenavir, saquinavir, or tipranavir due to lack of exposure data. The use of cobicistat is not recommended with darunavir 600 mg twice daily, fosamprenavir, saquinavir or tipranavir. Complex or unknown mechanisms of drug interactions preclude extrapolation of ritonavir drug interactions to certain cobicistat interactions. Cobicistat and ritonavir when administered with either atazanavir or darunavir may result in different drug interactions when used with concomitant medications. FDA Label Tybost is indicated as a pharmacokinetic enhancer of atazanavir 300 mg once daily or darunavir 800 mg once daily as part of antiretroviral combination therapy in human immunodeficiency virus-1 (HIV-1) infected adults and adolescents aged 12 years and older: weighing at least 35Â kg coâadministered with atazanavir orweighing at least 40Â kg coâadministered with darunavir. Treatment of human immunodeficiency virus (HIV-1) infection Mechanism of Action Cobicistat is a mechanism-based inhibitor of cytochrome P450 3A (CYP3A) isoforms. Inhibition of CYP3A-mediated metabolism by cobicistat increases the systemic exposure of CYP3A substrates atazanavir and darunavir and therefore enables increased anti-viral activity at a lower dosage. Cobicistat does not have any anti-HIV activity on its own. Cobicistat is a mechanism-based CYP3A inhibitor designed as a pharmacoenhancer to boost pharmacokinetics of HIV protease inhibitors [1]. FDA-approved as part of combination regimens (e.g., with elvitegravir) for HIV-1 infection treatment [2]. Unlike ritonavir, it lacks anti-HIV activity and shows improved selectivity for CYP3A over other CYP isoforms [1][2]. |
| 分子式 |
C40H53N7O5S2
|
|
|---|---|---|
| 分子量 |
776.02
|
|
| 精确质量 |
775.354
|
|
| 元素分析 |
C, 61.91; H, 6.88; N, 12.63; O, 10.31; S, 8.26
|
|
| CAS号 |
1004316-88-4
|
|
| 相关CAS号 |
|
|
| PubChem CID |
25151504
|
|
| 外观&性状 |
White to yellow solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
974.5±65.0 °C at 760 mmHg
|
|
| 闪点 |
543.2±34.3 °C
|
|
| 蒸汽压 |
0.0±0.3 mmHg at 25°C
|
|
| 折射率 |
1.595
|
|
| LogP |
4.77
|
|
| tPSA |
194.5
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
10
|
|
| 可旋转键数目(RBC) |
20
|
|
| 重原子数目 |
54
|
|
| 分子复杂度/Complexity |
1120
|
|
| 定义原子立体中心数目 |
3
|
|
| SMILES |
CC(C)C1=NC(=CS1)CN(C)C(=O)N[C@@H](CCN2CCOCC2)C(=O)N[C@H](CC[C@H](CC3=CC=CC=C3)NC(=O)OCC4=CN=CS4)CC5=CC=CC=C5
|
|
| InChi Key |
ZCIGNRJZKPOIKD-CQXVEOKZSA-N
|
|
| InChi Code |
InChI=1S/C40H53N7O5S2/c1-29(2)38-43-34(27-53-38)25-46(3)39(49)45-36(16-17-47-18-20-51-21-19-47)37(48)42-32(22-30-10-6-4-7-11-30)14-15-33(23-31-12-8-5-9-13-31)44-40(50)52-26-35-24-41-28-54-35/h4-13,24,27-29,32-33,36H,14-23,25-26H2,1-3H3,(H,42,48)(H,44,50)(H,45,49)/t32-,33-,36+/m1/s1
|
|
| 化学名 |
thiazol-5-ylmethyl ((2R,5R)-5-((S)-2-(3-((2-isopropylthiazol-4-yl)methyl)-3-methylureido)-4-morpholinobutanamido)-1,6-diphenylhexan-2-yl)carbamate
|
|
| 别名 |
Cobicistat; 1004316-88-4; cobicistatum; CHEBI:72291; LW2E03M5PG; COBICISTAT, (R,R,S)-; GS9350; trade name: Tybost; Genvoya; GS-9350; GS 9350;
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (2.68 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (2.68 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (2.68 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5% DMSO+40% PEG 300+ddH2O: 30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.2886 mL | 6.4431 mL | 12.8863 mL | |
| 5 mM | 0.2577 mL | 1.2886 mL | 2.5773 mL | |
| 10 mM | 0.1289 mL | 0.6443 mL | 1.2886 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05748093 | Recruiting | Drug: Cobicistat | Non-small Cell Lung Cancer | Maastricht University Medical Center | April 1, 2024 | Phase 4 |
| NCT02503462 | Terminated | Drug: ritonavir Drug: cobicistat |
AIDS-related Dementia Complex | University Hospital, Basel, Switzerland | July 2015 | Phase 4 |
| NCT03858491 | Completed | Drug: Cobicistat | Non Small Cell Lung Cancer | Academisch Ziekenhuis Maastricht | November 1, 2020 | Early Phase 1 |
| NCT04322214 | Completed | Drug: Cobicistat 150 MG Drug: Placebo |
Pharmacokinetic Interactions HIV |
Fundación FLS de Lucha Contra el Sida, las Enfermedades Infecciosas y la Promoción de la Salud y la Ciencia |
January 30, 2020 | Phase 1 |