| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 100mg |
|
||
| 200mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
Microtubule/Tubulin
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:秋水仙碱通过与微管的主要成分可溶性微管蛋白异二聚体结合来发挥其生物学作用。总结了多种来源的微管蛋白的秋水仙碱结合能力,深入探讨了秋水仙碱与脑微管蛋白结合的机制。秋水仙素结构与微管蛋白结合活性之间的关系提供了对负责与微管蛋白高亲和力结合的秋水仙碱结构特征的深入了解,并对秋水仙碱系列中的类似物进行了审查。根据结合机制描述和评估了缔合的热力学和动力学方面。秋水仙碱与微管蛋白的结合导致秋水仙碱低能电子光谱的异常改变。根据秋水仙碱-微管蛋白复合物的性质讨论了与微管蛋白结合的秋水仙碱的光谱特征。尝试定位微管蛋白上的高亲和力秋水仙碱结合位点。给予吲哚美辛 24 小时后的损伤指数表明,秋水仙碱治疗可抑制吲哚美辛引起的小肠损伤达 86%(1 mg/kg)和 94%(3 mg/kg)。秋水仙碱抑制裂解的 caspase-1 和成熟 IL-1β 的蛋白表达,而不影响 NLRP3 和 IL-1β 的 mRNA 表达。激酶测定:暴露于 1μM 秋水仙碱(一种微管破坏剂)会引发大鼠小脑颗粒细胞 (CGC) 细胞凋亡。秋水仙碱治疗还会导致 Ca2+ 对化学去极化的反应发生改变,并导致静息细胞内 Ca2+ 浓度适度但进行性增加[1]。秋水仙碱通过与微管的主要成分可溶性微管蛋白异二聚体结合来发挥其生物学作用。总结了多种来源的微管蛋白的秋水仙碱结合能力,深入探讨了秋水仙碱与脑微管蛋白结合的机制。秋水仙素结构与微管蛋白结合活性之间的关系提供了对负责与微管蛋白高亲和力结合的秋水仙碱结构特征的深入了解,并对秋水仙碱系列中的类似物进行了审查。根据结合机制描述和评估了缔合的热力学和动力学方面。秋水仙碱与微管蛋白的结合导致秋水仙碱低能电子光谱的异常改变。根据秋水仙碱-微管蛋白复合物的性质讨论了与微管蛋白结合的秋水仙碱的光谱特征。尝试定位微管蛋白上的高亲和力秋水仙碱结合位点[2]。给予吲哚美辛 24 小时后的损伤指数表明,秋水仙碱治疗可抑制吲哚美辛引起的小肠损伤达 86%(1 mg/kg)和 94%(3 mg/kg)。秋水仙碱抑制裂解的 caspase-1 和成熟 IL-1β 的蛋白表达,而不影响 NLRP3 和 IL-1β 的 mRNA 表达。细胞测定:
|
| 体内研究 (In Vivo) |
在吲哚美辛给药前 30 分钟口服秋水仙碱 (1 mg/kg)。给予吲哚美辛24小时后,秋水仙碱处理的小鼠小肠中伊文思蓝染色的病变比载体处理的小鼠小。此外,组织学检查表明,与媒介物处理的小鼠相比,秋水仙碱处理的小鼠粘膜炎症和溃疡较少,病变的大小和数量减少。与赋形剂治疗相比,秋水仙碱治疗在 1 mg/kg 和 3 mg/kg 剂量下显着降低病变指数(分别降低 86% 和 94%)。秋水仙碱处理在 1 mg/kg 和 3 mg/kg 剂量下显着抑制成熟 IL-1β 的蛋白水平(分别抑制 56% 和 69%),而不影响 pro-IL-1β 的蛋白水平。秋水仙碱处理还在 1 mg/kg 和 3 mg/kg 剂量下显着抑制裂解的 caspase-1 的蛋白水平(分别抑制 26% 和 39%),而不影响 pro-caspase-1 的蛋白水平。
秋水仙碱治疗对吲哚美辛诱导的小肠损伤的预防作用[3] 我们证实,不含吲哚美辛的赋形剂治疗不会提前导致小肠损伤。在吲哚美辛给药前30分钟口服赋形剂或秋水仙碱(1mg/kg)。服用吲哚美辛24小时后,Colchicine/秋水仙碱治疗的小鼠小肠中伊文思蓝染色的病变比赋形剂治疗的小鼠小。此外,组织学检查显示,与赋形剂治疗的小鼠相比,秋水仙素治疗的小鼠的粘膜炎症和溃疡较少,病变的大小和数量也有所减少(图2a)。与赋形剂治疗相比,秋水仙碱治疗在1 mg/kg和3 mg/kg剂量下显著降低了病变指数(分别降低了86%和94%)(图2b) 接下来,我们研究了秋水仙碱对吲哚美辛给药后6小时炎性小体成分mRNA水平的影响。与赋形剂治疗相比,Colchicine/秋水仙碱治疗在1 mg/kg和3 mg/kg的剂量下没有显著改变NLRP3(图2c)、IL-1β(图2d)或胱天蛋白酶-1(图2e)的mRNA水平。 此外,我们研究了秋水仙素治疗对吲哚美辛给药后6小时成熟IL-1β(p17)和切割型半胱氨酸天冬氨酸蛋白酶-1(p10)蛋白表达的影响。秋水仙素治疗在1mg/kg和3mg/kg的剂量下显著抑制了成熟IL-1β的蛋白质水平(分别降低了56%和69%),而不影响前IL-1β的蛋白水平。秋水仙素治疗在1 mg/kg和3 mg/kg的剂量下也显著抑制了切割的胱天蛋白酶-1的蛋白质水平(分别降低了26%和39%),而不影响前胱天蛋白酶1的蛋白质水平。(图2f-j)。 秋水仙素治疗对受损小肠中切割胱天蛋白酶-1表达和定位的预防作用[3] 使用免疫荧光测定切割的胱天蛋白酶-1蛋白的定位和表达水平,结果表明切割的胱天蛋白酶-1在小肠黏膜固有层中广泛表达6 服用吲哚美辛后h,而用Colchicine/秋水仙素治疗的小鼠表现出切割的胱天蛋白酶-1表达的显著降低(图3a)。用CD68对切割的胱天蛋白酶-1进行双重染色表明,表达切割的胱天蛋白酶-1的大多数细胞是巨噬细胞和单核细胞(图3b)。 外源性IL-1β和Colchicine/秋水仙素治疗对吲哚美辛诱导的小肠损伤的影响[3] 为了研究IL-1β在非甾体抗炎药诱导的小肠损伤发展中的作用,小鼠在吲哚美辛治疗后3小时接受小鼠重组IL-1β(0.1μg/kg)的载体或腹腔注射。在吲哚美辛激发前给予重组IL-1β在宏观(即损伤指数)和微观评估中消除了秋水仙素对吲哚美痛诱导的损伤的预防作用,但补充IL-1β不影响载体治疗小鼠的损伤严重程度(图4a,b)。 秋水仙素治疗的预防作用是通过抑制NLRP3炎性小体来介导的[3] 确认NLRP3炎性小体/半胱氨酸天冬氨酸蛋白酶-1/IIL-1β轴参与Colchicine/秋水仙素、赋形剂或秋水仙素(1或3 mg/kg)在给予吲哚美辛之前给予NLRP3基因紊乱的小鼠(NLRP3-/-小鼠)。与之前的研究一致23,吲哚美辛诱导的NLRP3-/-小鼠小肠损伤在宏观上很轻微。秋水仙碱治疗并没有进一步抑制NLRP3-/-小鼠的小肠损伤(图5a)。与野生型小鼠相比,使用损伤指数评估的吲哚美辛诱导的NLRP3-/-小鼠小肠损伤显著减少了77%,我们证实,1 mg/kg和3 mg/kg剂量的秋水仙碱治疗未能抑制NLRP3-/-小鼠的小肠损伤(图5b)。 我们研究了秋水仙素治疗(1 mg/kg和3 mg/kg)对NLRP3-/-小鼠炎症组分mRNA水平的影响。与野生型小鼠相比,NLRP3-/-小鼠的IL-1β(图5c)和胱天蛋白酶-1(图5d)的mRNA水平没有显著变化。在NLRP3-/-小鼠中,与赋形剂治疗相比,1 mg/kg和3 mg/kg剂量的秋水仙素治疗没有显著改变IL-1β(图5c)和胱天蛋白酶-1(图5d)的mRNA水平 我们研究了秋水仙素治疗对NLRP3-/-小鼠成熟IL-1β(p17)和切割型半胱氨酸天冬氨酸蛋白酶-1(p10)蛋白表达的影响。与野生型小鼠相比,NLRP3-/-小鼠中成熟IL-1β和切割的胱天蛋白酶-1的蛋白质水平分别显著降低了33%和57%。与野生型小鼠相比,NLRP3-/-小鼠中pro-IL-1β和pro-capase-1的蛋白质水平没有变化。这些蛋白质的表达水平在接受秋水仙素治疗和不接受秋水仙碱治疗的NLRP3-/-小鼠中相似(图5e-i)。 |
| 酶活实验 |
在1μM的时间里,在大鼠小脑颗粒细胞(CGC)中,微管破坏剂秋水仙碱引起细胞凋亡。此外,施用秋水仙碱会导致静息细胞内 Ca2+ 浓度逐渐但适度升高,以及 Ca2+ 对化学去极化的反应发生变化 [...]。通过与可溶性微管蛋白异二聚体(微管的主要组成部分)结合,秋水仙碱具有其生物学效应。对秋水仙碱与脑微管蛋白结合的机制进行了广泛的检查,并总结了微管蛋白结合所有来源的秋水仙碱的能力。通过秋水仙素类结构与微管蛋白结合活性之间的相关性,可以深入了解秋水仙碱使其能够与微管蛋白高亲和力结合的结构特征。秋水仙碱系列类似物也检验了这种关系。结合机制讨论和评估了缔合的动力学和热力学特征。秋水仙碱的低能电子光谱在与微管蛋白结合后表现出特殊的变化。结合秋水仙碱与微管蛋白结合的光谱特征讨论了秋水仙碱-微管蛋白复合物的性质。人们尝试鉴定微管蛋白的高亲和力秋水仙碱结合位点[2]。给予吲哚美辛24小时后的损伤指数表明,秋水仙碱治疗可抑制86%(1mg/kg)和94%(3mg/kg)吲哚美辛引起的小肠损伤。秋水仙碱在不影响 NLRP3 和 IL-1β mRNA 表达的情况下,抑制成熟 IL-1β 和裂解 caspase-1 的蛋白表达。
秋水仙素通过与微管的主要成分可溶性微管蛋白异二聚体结合来发挥其生物学作用。总结了各种来源的微管蛋白的秋水仙素结合能力,并深入探讨了秋水仙素与脑微管蛋白结合的机制。秋水仙素类结构和微管蛋白结合活性之间的关系提供了对秋水仙素与微管蛋白高亲和力结合的结构特征的深入了解,并对秋水仙碱系列中的类似物进行了综述。根据结合机制描述和评估了缔合的热力学和动力学方面。秋水仙素与微管蛋白的结合导致秋水仙素的低能电子光谱发生异常变化。根据秋水仙素-微管蛋白复合物的性质,讨论了秋水仙素与微管蛋白结合的光谱特征。本文介绍了定位微管蛋白上高亲和力秋水仙素结合位点的尝试[2]。 |
| 细胞实验 |
HeLa 细胞在 6 孔板中生长,两小时后用 100 μM EBI 处理。之后,用不同浓度的 KXO1、长春花碱或秋水仙碱处理它们。使用radioimmuno_x005f?沉淀测定缓冲液提取总蛋白后,使用Western blot分析β~-微管蛋白。上样对照是 GAPDH。进行蛋白质印迹过程。
电生理学[4] 在室温(20-24°C)下,在-60 mV的保持电位下,在全细胞电压钳配置中记录EGFP阳性转染的HEK 293细胞的甘氨酸诱发电流(Lara等人,2019)。贴片电极(3-4 mΩ)从硼硅酸盐玻璃中拔出,并填充(单位为mM):120 CsCl、8 EGTA、10 HEPES(pH 7.4)、4 MgCl2、0.5 GTP和2 ATP。外部溶液含有(以mM计)140 NaCl、5.4 KCl、2.0 CaCl2、1.0 MgCl2、10 HEPES(pH 7.4)和10葡萄糖。使用Axoclamp 200B放大器进行全细胞记录,并使用Clampex 10.1或Axopatch 10.0软件采集。使用Clampfit 10.1离线进行数据分析。使用手动施加激动剂脉冲(3-4s)和定制设计的重力馈送微灌注系统的出口管(内径200μm)获得外源性甘氨酸诱发电流,该系统位于距离记录细胞50-100μm的位置。用于细胞附着配置中α3GlyR单通道记录的方法先前已发表(Marabelli等人,2013;Lara等人,2019)。贴片移液管的尖端电阻为10-20mΩ,并在微锻件中手动火抛光。数据经过滤波(1kHz低通8极巴特沃斯),并使用Axopatch 200B放大器和1322A Digidata在5-20kHz下采集。使用pClamp软件获取数据,并使用Clampfit 10.1进行离线分析秋水仙素原液在高纯度蒸馏水中制备,随后在实验当天稀释到记录溶液中。在全细胞实验中,使用手动施加脉冲(1-2s)将秋水仙素与甘氨酸共同施加。在细胞附着记录中,将Colchicine与甘氨酸一起应用于移液管内溶液。 暴露于1微摩尔的Colchicine(一种微管破坏剂)会引发大鼠小脑颗粒细胞(CGC)的凋亡。12小时后开始出现凋亡核,随后出现寡核苷酸DNA梯状条带,而当大多数细胞已经有凋亡核时,线粒体3-(4,5-二甲基噻唑-2-基)-2,5-二苯基四氮唑溴化物代谢的抑制在18至24小时之间变得显著。在这些事件之前,tau蛋白丢失,α和β微管蛋白断裂秋水仙碱治疗还导致Ca2+对化学去极化反应的改变,以及静息细胞内Ca2+浓度的中度但渐进性增加。Colchicine处理后,几乎所有的神经元都表达了c-Fos。然而,尽管在部分细胞群中c-Fos水平随后下降,但在经历凋亡的神经元中,该蛋白仍然表达,但细胞内定位异常。在12小时时也检测到组成型一氧化氮合酶(NOS-I)的表达增加,随后亚硝酸盐的产生增加。用100nM紫杉醇处理以稳定微管,可防止秋水仙素诱导的DNA梯状条带和凋亡体形成。相比之下,用N-甲基-D-天冬氨酸受体拮抗剂MK-801或L型Ca2+通道阻断剂预处理并不能阻止秋水仙碱诱导的CGC凋亡。NOS抑制剂在防止凋亡小体形成和DNA梯状条带方面也无效,但它们延迟了二次细胞裂解。这些结果支持秋水仙素诱导的细胞骨架改变直接引发导致CGC凋亡的遗传和结构修饰的观点[1]。 |
| 动物实验 |
Mice: Male 8-week-old mice that are free of specific pathogens are used. Both NLRP3?/? mice and wild-type C57BL/6 mice on a C57BL/6 background are employed. 30 minutes before indomethacin is given, either a vehicle or 1 or 3 mg/kg of Colchicine is given orally to investigate the impact of Colchicine on NSAID-induced small intestinal damage. Three hours after being treated with indomethacin, mice were given intraperitoneal injections of either mouse recombinant IL-1β (0.1 μg/kg) or sterilized phosphate buffered saline. Before indomethacin is given to NLRP3?/? mice, vehicle or colchicine (1 or 3 mg/kg) is also given. 24 hours after indomethacin is administered, the lesion index is assessed, and 6 hours later, the mRNA and protein expression of inflammasome components is investigated.
To examine the effects of colchicine on NSAID-induced small intestinal injury, vehicle or colchicine (1 or 3 mg/kg; Wako Pure Chemical Industries, Ltd., Kyoto, Japan) was administered orally 30 min prior to indomethacin administration. Mice received intraperitoneal injections of sterilized phosphate buffered saline or mouse recombinant IL-1β (0.1 μg/kg; R&D Systems, Inc., Minneapolis, MN) 3 h after indomethacin treatment. Vehicle or colchicine (1 or 3 mg/kg) was also administered to NLRP3−/− mice before indomethacin administration. We evaluated the lesion index 24 h after indomethacin administration, and examined mRNA and protein expression of inflammasome components 6 h after indomethacin administration.[3] The inflammasome is a large, multiprotein complex that consists of a nucleotide-binding oligomerization domain-like receptor (NLR), an apoptosis-associated speck-like protein containing a caspase recruitment domain, and pro-caspase-1. Activation of the inflammasome results in cleavage of pro-caspase-1 into cleaved caspase-1, which promotes the processing of pro-interleukin (IL)-1β into mature IL-1β. We investigated the effects of colchicine on non-steroidal anti-inflammatory drug (NSAID)-induced small intestinal injury and activation of the NLR family pyrin domain-containing 3 (NLRP3) inflammasome. Colchicine treatment inhibited indomethacin-induced small intestinal injury by 86% (1 mg/kg) and 94% (3 mg/kg) as indicated by the lesion index 24 h after indomethacin administration. Colchicine inhibited the protein expression of cleaved caspase-1 and mature IL-1β, without affecting the mRNA expression of NLRP3 and IL-1β. Although treatment with recombinant IL-1β (0.1 μg/kg) did not change the severity of small intestinal damage, the preventive effects of colchicine were abolished by supplementation with the same dose of recombinant IL-1β. Indomethacin-induced small intestinal damage was reduced by 77%, as determined by the lesion index in NLRP3(-/-) mice, and colchicine treatment failed to inhibit small intestinal damage in NLRP3(-/-) mice. These results demonstrate that colchicine prevents NSAID-induced small intestinal injury by inhibiting activation of the NLRP3 inflammasome. [3] Colchicine is a plant alkaloid that is widely used as a therapeutic agent. It is widely accepted that colchicine reduces the production of inflammatory mediators mainly by altering cytoskeleton dynamics due to its microtubule polymerization inhibitory activity. However, other lines of evidence have shown that colchicine exerts direct actions on the function of ion channels, which are independent of cytoskeleton alterations. Colchicine is able to modify the function of several pentameric ligand-gated ion channels, including glycine receptors (GlyRs). Previous electrophysiological studies have shown that colchicine act as an antagonist of GlyRs composed by the α 1 subunit. In addition, it was recently demonstrated that colchicine directly bind to the α 3 subunit of GlyRs. Interestingly, other studies have shown a main role of α 3GlyRs on chronic inflammatory pain. Nevertheless, the functional effects of colchicine on the α 3GlyR function are still unknown. Here, by using electrophysiological techniques and bioinformatics, we show that colchicine inhibited the function of the α 3GlyRs. Colchicine elicited concentration-dependent inhibitory effects on α 3GlyRs at micromolar range and decreased the apparent affinity for glycine. Single-channel recordings show that the colchicine inhibition is associated with a decrease in the open probability of the ion channel. Molecular docking assays suggest that colchicine preferentially bind to the orthosteric site in the closed state of the ion channel. Altogether, our results suggest that colchicine is a competitive antagonist of the α 3GlyRs. [4] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Colchicine is rapidly absorbed after oral administration from the gastrointestinal tract. The bioavailability of colchicine is about 45%: one study suggests that colchicine bioavailability is highly variable, ranging from 24 to 88%. In healthy adults, the mean Cmax of 2.5 ng/mL (range 1.1 to 4.4 ng/mL) was achieved in one to two hours (range 0.5 to 3 hours) after a single dose administered under fasting conditions. In a multiple-dose study of colchicine administration at a dose of 1 mg per day, steady-state concentrations were achieved by day 8 following administration. Administration of colchicine with food does not affect the colchicine absorption rate but decreases the extent of colchicine by approximately 15%. In a pharmacokinetic study of healthy research subjects who received 1 mg of oral colchicine, about 40% to 65% of the dose was recovered in the urine in the form of an unchanged drug. Colchicine undergoes enterohepatic recirculation and biliary excretion. The mean apparent volume of distribution in young and healthy patients is about 5-8 L/kg. It is known to cross the placenta and distribute into the breast milk. Colchicine has been found to distribute to various tissues, mainly into the bile, liver, and kidney tissues. Smaller amounts have been detected in the heart, lungs, intestinal tissue, and stomach. In one pharmacokinetic study involving patients who received a single oral dose of 0.6 mg colchicine, the clearance was 0.0321 ± 0.0091 mL/min in young, healthy adults and 0.0292 ± 0.0071 mL/min in adults between the ages of 60 and 70 years. Patients with end-stage renal impairment showed a 75% lower clearance of colchicine. In a pharmacokinetic study of patients with Familial Mediterranean Fever (FMF), the apparent mean clearance was calculated at 0.726 ± 0.110 L/h/kg. The absorption of colchicine is rapid but variable. Peak plasma concentrations occur 0.5 to 2 hours after dosing. In plasma, 50% of colchicine is protein-bound. There is significant enterohepatic circulation. The exact metabolism of colchicine is unknown but seems to involve deacetylation by the liver. Only 10% to 20% is excreted in the urine, although this increases in patients with liver disease. The kidney, liver, and spleen also contain high concentrations of colchicine, but it apparently is largely excluded from heart, skeletal muscle, and brain. The plasma half-life of colchicine is approximately 9 hours, but it can be detected in leukocytes and in the urine for at least 9 days after a single intravenous dose. ... Two cases involving suicide by the ingestion of medications marketed in France /is reported/. In case 1, only heart blood was taken after body external examination. In case 2 an autopsy was performed and heart blood, urine, gastric contents and bile were taken for toxicological analysis. Colchicine was assayed in biological specimens by an HPLC-DAD method, after extraction by dichloromethane at pH 8, adding prazepam as internal standard (IS). Analyses were performed on a Symetry C-8 column. Mobile phase was a gradient of acetonitrile/pH 3.8 phosphate buffer. Colchicine is eluted at 13.1 min and the method is linear for blood, urine and bile over the range 4-1000 ng/mL. LOQ is 4 ng/mL. The concentrations of colchicine detected are: case 1: heart blood 13 ng/mL; case 2: heart blood 66 ng/mL, urine 500 ng/mL, gastric content 12 ng/mL, bile 5632 ng/mL. Our findings are in the range of lethal concentrations previously described, but there is no correlation with the amount of ingested drug. Even after massive overdose, it could be impossible to detect colchicine in blood, and as there is a widespread enterohepatic recirculation before excretion in bile and feces, bile is the target sample to analyse. We conclude in both cases that the cause of death was suicide with colchicine. It appears very important to perform an autopsy in order to obtain bile, urine, heart blood and femoral blood. After oral administration plasma concentrations reach a peak within 0.5 to 2 hours and afterwards decrease rapidly within 2 hours. The plasma half-life is 60 minutes. Colchicine may remain in tissues for as long as 10 days. Information was available on urinary excretion in 5 cases. Concentrations in urine are 10 to 80 fold higher than those in plasma. Four to 25 per cent of the dose ingested was excreted in urine over three to ten days. Excretion was specially high during the first 24 hours following ingestion. Colchicine is eliminated in urine up to the tenth day. For more Absorption, Distribution and Excretion (Complete) data for COLCHICINE (12 total), please visit the HSDB record page. Metabolism / Metabolites Colchicine is metabolized in the liver. It undergoes CYP3A4-mediated demethylation into major metabolites, 2-O-demethylcolchicine and 3-O-demethylcolchicine. It also forms one minor metabolite, 10-O-demethylcolchicine ([colchiceine]). Plasma levels of these metabolites are less than 5% of parent drug. Colchicine undergoes some hepatic metabolism. Colchicine is partially deacetylated in the liver. Large amounts of colchicine and of its metabolites undergo enterohepatic circulation. This may explain the occurrence of a second plasma peak concentration observed 5 to 6 hours after ingestion. Three novel conjugation metabolites of colchicine were identified in rat bile facilitated by enhanced on-line liquid chromatography-accurate radioisotope counting. The known 2- and 3-demethylcolchicines (DMCs) underwent O-sulfate conjugation in addition to the previously described O-glucuronidation. 2-DMC was preferably O-glucuronidated, whereas 3-DMC predominantly yielded O-sulfation conjugates, indicating phase II conjugation regiopreferences. Moreover, M1 was identified as a novel glutathione conjugate and a possible biotransformation pathway for its formation was proposed. The known 2-DMC (M6), 3-DMC (M7), 2-DMC glucuronide (M4), and novel 3-DMC sulfate (M3) were confirmed as the major metabolites. ... Biological Half-Life After several doses of 0.6 mg twice daily, the average elimination half-life of colchicine ranges from 26.6 to 31.2 hours. Another study reported the elimination half-life ranging to be 20 to 40 hours. Following IV administration of a single therapeutic dose (as of August 2008, IV preparations are no longer commercially available in the US), colchicine is rapidly removed from the plasma; plasma half-life is about 20 minutes. The drug has a half-life of about 60 hours in leukocytes. The elimination half life is variable, ranging from 4.4 hours in normal patients to 30 hours or more in elderly patients. A therapeutic dose produced a half life of 18.8 hours in patients with renal dysfunction. After a single 2 mg intravenous dose the average plasma half life is 20 minutes. Plasma half-life is increased in severe renal disease (40 min) and decreased in severe hepatic disease (9 min). |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION: Colchicine is an antigout preparations. Colchicine is available as tablets and, in some countries, as injectable solutions. Colchicine is an alkaloid of Colchicum autumnale (autumn crocus, meadow saffron). Colchicum is also present in Gloriosa superba. Colchicine is a pale yellow odorless powder or scales. It darkens on exposure to light. Colchicine is used for acute gout attacks to reduce pain and inflammation. It may be used on long-term basis to prevent or reduce the frequency of attacks. Colchicine is used on long-term basis to prevent fever and recurrent polyserositis. Colchicine is effective in preventing the amyloidosis in this condition. Colchicine has been showed to be effective in the treatment of articular, cutaneous and mucosal symptoms. Colchicine has been used in the treatment of scleroderma and sarcoidosis. HUMAN EXPOSURE: Main risks and target organs: Colchicine exerts a multiorgan toxicity. The main toxic effects are related to the effects of colchicine on cellular division and account for diarrhea, bone marrow depression, alopecia. Other acute effects are hypovolemia, shock, and coagulation disturbances, which may lead to death. Summary of clinical effects: Toxic manifestations appear after a delay of 2 to 12 hours following ingestion or parenteral administration. Symptomatology progresses in three stages: Stage I (Day 1 to 3) gastrointestinal and circulatory phase: Severe gastrointestinal irritation: Nausea, vomiting, abdominal cramps, severe diarrhea. Dehydration, hypovolemia, shock. Cardiogenic shock may occur and may result in death within the first 72 hours. Hypoventilation, acute respiratory distress syndrome. Stage II (Day 3 to 10) bone marrow aplasia phase: Bone marrow aplasia with agranulocytosis. Coagulation disorders with diffuse hemorrhages. Rhabdomyolysis, polyneuritis, myopathy, acute renal failure and infections. Stage III: (After 10 day) recovery phase: Alopecia. Routes of entry: Oral: Oral absorption is the most frequent cause of intoxication. Parenteral: Intoxications after parenteral administration are rare, however, the toxic dose appears to be lower than the oral toxic dose. A fatal bone marrow aplasia in a 70 year-old man after 10 mg intravenous colchicine over 5 days. Intoxication with multisystemic reactions after instillation of colchicine into the penile urethra for treatment of condyloma acuminata. Absorption by route of exposure: Oral: Rapidly absorbed from the gastro-intestinal tract. Peak plasma concentration is reached 0.5 to 2 hours after ingestion. Half time of absorption is 15 minutes. Absorption may be modified by pH, gastric contents, intestinal motility. Colchicine is not totally absorbed. There is an important hepatic first pass effect. Colchicine distributes in a space larger than that of the body. In severe renal or liver diseases the volume of distribution is smaller. Colchicine accumulates in kidney, liver, spleen, gastro-intestinal wall and leucocytes and is apparently excluded in heart, brain, skeletal muscle. Colchicine crosses the placenta and has also been found in maternal milk. Biological half-life by route of exposure: Parenteral: After a single 2 mg intravenous dose the average plasma half-life is 20 minutes. Plasma half-life is increased in severe renal disease (40 min) and decreased in severe hepatic disease (9 min). Oral: After oral administration plasma concentrations reach a peak within 0.5 to 2 hours and afterwards decrease rapidly within 2 hours. The plasma half-life is 60 minutes. Colchicine may remain in tissues for as long as 10 days. Metabolism: Colchicine undergoes some hepatic metabolism. Colchicine is partially deacetylated in the liver. Large amounts of colchicine and of its metabolites undergo enterohepatic circulation. This may explain the occurrence of a second plasma peak concentration observed 5 to 6 hours after ingestion. Elimination by route of exposure: Colchicine is excreted unchanged (10 to 20 percent) or as metabolites. Oral: Urinary excretion amount to 16 to 47% of an administered dose. 50 to 70% of colchicine is excreted unchanged and 30 to 50% as metabolites. 20% of the dose administered is excreted in urine in the first 24 hours and 27.5% in the first 48 hours. Colchicine is detected in urine up to 7 to 10 days after ingestion. Urinary excretion is increased in patients with impaired hepatic function. Bile: 10 to 25% of colchicine is excreted in the bile. Feces: Large amounts of the drug are excreted in the feces. Breast Milk: Colchicine may be eliminated in breast milk. Intravenous: Feces: After intravenous administration 10 to 56% is excreted in the feces within the first 48 hours. Breast Milk: Colchicine may be eliminated in breast milk. Mode of action: Colchicine binds to tubulin and this prevents its polymerization into microtubules. The binding is reversible and the half-life of the colchicine-tubulin complex is 36 hours. Colchicine impairs the different cellular functions of the microtubule: separation of chromosome pairs during mitosis (because colchicine arrests mitosis in metaphase), ameboid movements, phagocytosis. Mitosis blockade accounts for diarrhoea, bone marrow depression and alopecia. Colchicine may have a direct toxic effect on muscle, peripheral nervous system and liver. Inhibition of cellular function does not, however, account for all the organ failures seen in severe overdose. Pharmacodynamics: Gout inflammation is initiated by urate crystals within tissues. The crystals are ingested by neutrophils but this leads to the release of enzymes and the destruction of the cells. Chemotactic factors are released and attract more neutrophils. Colchicine may act by preventing phagocytosis, the release of chemotactic factors and the response of neutrophils. Colchicine has other properties such as antipyretic effects, respiratory depression, vasoconstriction and hypertension. Adults: Oral: The severity and the mortality rate of the poisoning is directly related to the dose ingested. Intravenous: A fatal bone marrow aplasia in a 70-year-old patient is reported. The enhanced toxicity of intravenous colchicine is probably due to the higher bioavailability of colchicine after parenteral administration. Teratogenicity: Colchicine is contraindicated in pregnancy as Down's syndrome and spontaneous abortion have been reported. Colchicine should be discontinued three months prior to conception. Interactions: A case of acute cyclosporin nephrotoxicity induced by colchicine administration has been reported. Colchicine may interfere with cyclosporin pharmacokinetics by increasing cyclosporin plasma levels either by enhancing cyclosporin absorption or by reducing its hepatic metabolism. Main adverse effects: Gastrointestinal symptoms are a common complication of chronic colchicine therapy. Fatal outcomes have been reported after intravenous colchicine therapy. Gastrointestinal: vomiting, diarrhoea, abdominal discomfort, paralytic ileus, malabsorption syndrome with steatorrhea. Hematological: Bone marrow depression with agranulocytosis, acute myelomonocytic leukaemia, multiple myeloma, thrombocytopenia. Neurological: Peripheral neuritis, myopathy and rhabdomyolysis. Dermatological: Allergic reactions are rare urticaria; oedema may be seen. Alopecia has been reported after chronic treatment. Reproductive system: A reversible, complete azoospermia has been reported. Metabolic: Colchicine is capable of producing a reversible impairment of vitamin B12 absorption. Porphyria cutanea tarda has been reported. Others: Hyperglycemia has been reported in a 58-year-old woman who ingested colchicine and developed transient diabetes mellitus has been reported. Hyperlipemia: A transient hyperlipemia has been reported. Hyperuricemia: A transient hyperuricemia has also been noted. Hyperthermia-fever: Occurrence of fever may be relate to an infectious complication, especially during the stage of aplasia. Special risks: Pregnancy: Two cases of Down's syndrome babies have been reported. The obstetric histories of 36 women with familial Mediterranean fever on long-term colchicine treatment between 3 and 12 years have been reported. Seven of 28 pregnancies ended in miscarriage. Thirteen women had periods of infertility. All 16 infants born to mothers who had taken colchicine during pregnancy were healthy. The authors do not advise discontinuation of colchicine before planned pregnancy but recommend amniocentesis for karyotyping and reassurance. Breast-feeding: As colchicine is eliminated in the breast milk breast-feeding should be avoided. Interactions Colchicine has been shown to induce reversible malabsorption of vitamin B12, apparently by altering the function of ileal mucosa. Results of animal studies have suggested that colchicine may enhance response to sympathomimetic agents and CNS depressants. (1) Renal failure, either pre-existing or induced by a nephrotoxic drug, increases the risk of adverse effects in patients taking colchicine; (2) Combining colchicines with a macrolide (except for spiramycin) carries a risk of life-threatening pancytopenia; (3) Ciclosporin co-administration can aggravate the neuromuscular adverse effects of colchicine; (4) Combining colchicine with lipid-lowering drugs (statins and fibrates) can cause myopathy; (5) Several mechanisms have been implicated: competition for cytochrome P450 or P-glycoprotein, additive adverse effects (especially on muscle), and colchicine accumulation due to a reduction in its renal excretion; (6) Patients with gout should use colchicine only after failure of symptomatic treatment: ice application, paracetamol, and possibly ibuprofen, a nonsteroidal antiinflammatory drug with well-documented adverse effects; (7) If colchicine is nevertheless used, it should be at the minimum effective dose. Close clinical monitoring is required in order to detect early signs of adverse effects, especially diarrhea, the earliest sign in patients with renal failure and in the elderly. Colchicine and 3-hydroxy-3-methy-glutaryl coenzyme A (HMG-CoA) reductase inhibitors are well known to cause myopathy. Myotoxicity is dose-dependent in both drugs; therefore, the onset of symptoms usually takes months or years. We report the case of a patient with chronic renal failure who had been taking simvastatin for 2 years and developed acute weakness 2 weeks after the start of treatment with colchicines for recurrent gout. The electromyography and elevated muscle enzymes indicated that his symptoms were caused by myopathy. When this patient stopped taking both drugs, his weakness resolved rapidly. Acute myopathy induced by combination therapy with colchicines and simvastatin is rare. In patients with chronic renal failure, co-administration of colchicine with simvastatin may accelerate the onset of myopathy because CYP3A4 (part of cytochrome P450) is crucial in the breakdown of both drugs. When adding colchicine to a medication regimen that includes a HMG-CoA reductase inhibitor for patients with renal insufficiency, drugs that are metabolized outside the CYP3A4 system (e.g., fluvastatin and pravastatin) should be selected instead. For more Interactions (Complete) data for COLCHICINE (13 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Cat intravenous 0.25 mg/kg LD50 Mouse oral 5886 ug/kg LD50 Mouse ip 2 mg/kg LD50 Mouse iv 4.13 mg/kg For more Non-Human Toxicity Values (Complete) data for COLCHICINE (8 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Gout Suppressants Colchicine also is used in the prophylactic treatment of recurrent gouty arthritis. Colchicine has no effect on plasma concentrations or urinary excretion of uric acid; therefore, concomitant administration of allopurinol or a uricosuric agent (e.g., probenecid, sulfinpyrazone) is necessary to decrease serum urate concentrations. Prophylactic doses of colchicine should be administered before the initiation of allopurinol or uricosuric therapy because sudden changes in serum urate concentrations may precipitate acute gout attacks. After the serum urate concentration has been reduced to the desired level and acute gout attacks have not occurred for 3-6 months (some clinicians suggest 1-12 months), colchicine may be discontinued and the patient may be treated with urate lowering agents alone. Colchicine is frequently used in combination with probenecid to facilitate prophylactic therapy in patients with chronic gouty arthritis. The usefulness of the commercially available fixed-dosage preparation is limited, however, because the colchicine present exceeds the amount required by most patients. /Use Included in US product label/ Colchicine is used to relieve attacks of acute gouty arthritis. Nonsteroidal anti-inflammatory agents (NSAIAs) (e.g., indomethacin, ibuprofen, naproxen, sulindac, piroxicam, ketoprofen) are as effective as, and better tolerated than, usual dosages of colchicine for short-term use in relieving acute attacks of gouty arthritis. Corticosteroids also are used to relieve acute attacks of gouty arthritis. Colchicine is considered a second-line agent; colchicine may be used for the treatment of acute gouty arthritis in patients who have not responded to or who cannot tolerate recommended therapies (i.e., NSAIAs, corticosteroids). /Use Included in US product label/ 96 patients aged 15 yr or more with complete or incomplete Behcet's disease, whose visual acuity was 20/40 or less, and who had experienced at least 2 episodes of ocular attack during the 16 wk before the study were selected. 47 patients received cyclosporin (10 mg/kg) and 49 colchicine (1 mg/kg) daily for 16 wk. The frequency of ocular attack was reduced more in the cyclosporin group than in the colchicine group (p < 0.001). The severity of ocular attacks was also less severe after cyclosporin than after colchicine (p < 0.001). Colchicine alleviated oral aphthous ulcer in 10 patients (20%). Dermal lesions were alleviated in 15% of the colchicine group. Clinical symptoms were improved in 33% for the colchicine group, and 10 cases were aggravated. OKT4/OKT8 ratios were 1.44 in the colchicine group before the study and 1.46 after treatment. Frequently observed side effects of colchicine were hirsutism (2 patients) and renal dysfunction (2 patients). Treatment was stopped because of hepatic dysfunction in 2 colchicine cases. For more Therapeutic Uses (Complete) data for COLCHICINE (11 total), please visit the HSDB record page. Drug Warnings Colchicine injection has been available in the US since the 1950s and has been used for the treatment of acute attacks of gout. Colchicine injection preparations that have been commercially available have not been approved by the US Food and Drug Administration (FDA). Serious adverse events, some fatal, have been reported in patients receiving colchicine injection. Because of the potentially serious health risks associated with unapproved colchicine injection, FDA announced on February 8, 2008, that it would take enforcement action (e.g., seizure, injunction, other judicial proceeding) against all firms, including compounding pharmacies, attempting to manufacture, ship, or deliver colchicine injection. FDA will implement enforcement action against all firms attempting to manufacture or ship colchicine injection products that do not have a National Drug Code (NDC) number on or after February 8, 2008. For colchicine injection products with an NDC number, FDA will take enforcement action against all firms attempting to manufacture such products on or after March 10, 2008, and against firms that ship such products on or after August 6, 2008. Myelosuppression, leukopenia, granulocytopenia, thrombocytopenia, pancytopenia, and aplastic anemia with colchicine used in therapeutic doses have been reported. Colchicine-induced neuromuscular toxicity and rhabdomyolysis have been reported with chronic treatment in therapeutic doses. Patients with renal dysfunction and elderly patients, even those with normal renal and hepatic function, are at increased risk. The most common adverse reaction is diarrhea (23%). Pharyngolaryngeal pain was seen in 3% of patients treated for gout flares. Gastrointestinal tract adverse effects are the most frequent side effects in patients initiating colchicine, usually presenting within 24 hours, and occurring in up to 20% of patients given therapeutic doses. Typical symptoms include cramping, nausea, diarrhea, abdominal pain, and vomiting. These events should be viewed as dose-limiting if severe as they can herald the onset of more significant toxicity. For more Drug Warnings (Complete) data for COLCHICINE (13 total), please visit the HSDB record page. Pharmacodynamics Colchicine ameliorates the symptoms of gout and Familial Mediterranean fever. It possesses anti-inflammatory, anti-fibrotic, and cardiovascular protective effects. Colchicine was shown to exhibit anticancer properties, such as the inhibition of cancer cell migration and angiogenesis. Colchicine has a narrow therapeutic window. The inflammasome is a large, multiprotein complex that consists of a nucleotide-binding oligomerization domain-like receptor (NLR), an apoptosis-associated speck-like protein containing a caspase recruitment domain, and pro-caspase-1. Activation of the inflammasome results in cleavage of pro-caspase-1 into cleaved caspase-1, which promotes the processing of pro-interleukin (IL)-1β into mature IL-1β. We investigated the effects of colchicine on non-steroidal anti-inflammatory drug (NSAID)-induced small intestinal injury and activation of the NLR family pyrin domain-containing 3 (NLRP3) inflammasome. Colchicine treatment inhibited indomethacin-induced small intestinal injury by 86% (1 mg/kg) and 94% (3 mg/kg) as indicated by the lesion index 24 h after indomethacin administration. Colchicine inhibited the protein expression of cleaved caspase-1 and mature IL-1β, without affecting the mRNA expression of NLRP3 and IL-1β. Although treatment with recombinant IL-1β (0.1 μg/kg) did not change the severity of small intestinal damage, the preventive effects of colchicine were abolished by supplementation with the same dose of recombinant IL-1β. Indomethacin-induced small intestinal damage was reduced by 77%, as determined by the lesion index in NLRP3(-/-) mice, and colchicine treatment failed to inhibit small intestinal damage in NLRP3(-/-) mice. These results demonstrate that colchicine prevents NSAID-induced small intestinal injury by inhibiting activation of the NLRP3 inflammasome.[3] |
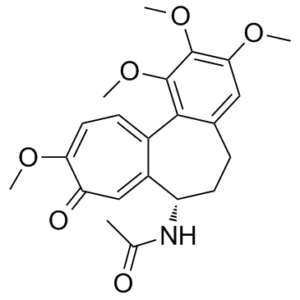
| 分子式 |
C22H25NO6
|
|
|---|---|---|
| 分子量 |
399.44
|
|
| 精确质量 |
399.168
|
|
| 元素分析 |
C, 66.15; H, 6.31; N, 3.51; O, 24.03
|
|
| CAS号 |
64-86-8
|
|
| 相关CAS号 |
Colchicine-d6;1217651-73-4;Colchicine-d3;1217625-62-1
|
|
| PubChem CID |
6167
|
|
| 外观&性状 |
White to light yellow solid powder
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
726.0±60.0 °C at 760 mmHg
|
|
| 熔点 |
150-160 °C (dec.)(lit.)
|
|
| 闪点 |
392.9±32.9 °C
|
|
| 蒸汽压 |
0.0±2.4 mmHg at 25°C
|
|
| 折射率 |
1.585
|
|
| LogP |
0.92
|
|
| tPSA |
83.09
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
29
|
|
| 分子复杂度/Complexity |
740
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
O(C([H])([H])[H])C1C(=C(C([H])=C2C=1C1=C([H])C([H])=C(C(C([H])=C1[C@]([H])(C([H])([H])C2([H])[H])N([H])C(C([H])([H])[H])=O)=O)OC([H])([H])[H])OC([H])([H])[H])OC([H])([H])[H]
|
|
| InChi Key |
IAKHMKGGTNLKSZ-INIZCTEOSA-N
|
|
| InChi Code |
InChI=1S/C22H25NO6/c1-12(24)23-16-8-6-13-10-19(27-3)21(28-4)22(29-5)20(13)14-7-9-18(26-2)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)/t16-/m0/s1
|
|
| 化学名 |
N-[(7S)-1,2,3,10-tetramethoxy-9-oxo-6,7-dihydro-5H-benzo[a]heptalen-7-yl]acetamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.78 mg/mL (6.96 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。 (<60°C).
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5035 mL | 12.5175 mL | 25.0350 mL | |
| 5 mM | 0.5007 mL | 2.5035 mL | 5.0070 mL | |
| 10 mM | 0.2504 mL | 1.2518 mL | 2.5035 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Effect of Colchicine on Progression of Known Coronary Atherosclerosis in Patients With Stable Coronary Artery Disease
CTID: NCT06342609
Phase: Phase 4 Status: Completed
Date: 2024-10-15
Preventive effects of colchicine treatment on indomethacin-induced small intestinal injury.Sci Rep. 2016; 6: 32587. |
|---|
Effect of exogenous IL-1β and colchicine treatment on indomethacin-induced small intestinal injury.Sci Rep. 2016; 6: 32587. |
Preventive effects of colchicine treatment are mediated by suppression of the NLRP3 inflammasome.Sci Rep. 2016; 6: 32587. |