| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
delta6D in ABMC-7 cells (IC50 = 0.015 μM); delta6D in Liver microsomes (IC50 = 0.56 μM); delta5D in ABMC-7 cells (IC50 = 0.67 μM); delta5D in ABMC-7 cells (IC50 = 3.4 μM)
|
|---|---|
| 体外研究 (In Vitro) |
CP-24879(盐酸盐)(0-10 μM,4 天)以浓度依赖性方式抑制 Δ6 + Δ5 去饱和酶活性,并导致 AA 浓度依赖性消耗和 LTC4 产量减少[1]。 CP-24879(盐酸盐)(0-10 μM,16 h)对肝细胞中油酸引起的甘油三酯积累具有抑制作用[2]。 CP-24879(盐酸盐)((0-10 μM,16 小时)以浓度依赖性方式抑制 LPS 诱导的炎症细胞因子的表达[2]。CP-24879(盐酸盐)(0-2 μM,4 小时)抑制去饱和酶活性并改善铁死亡[3]。
|
| 体内研究 (In Vivo) |
CP-24879(盐酸盐)(3 mg/kg,IP,每天 3 次,持续六或四天)抑制体内 Δ6 + Δ5 去饱和酶活性,消耗饲料小鼠肝脏中的 AA,同时防止小鼠肝脏中的 AA 补充。患有 EFAD 的小鼠[1]。 CP-24879(盐酸盐)(33 mg/kg,静脉注射,一次)清除速度相当快,半衰期相对较短[1]。
|
| 酶活实验 |
CP-24879(对异苯氧基苯胺)是一种苯胺衍生物,在筛选化学和天然产物库时被鉴定为混合delta5/delta6脱饱和酶抑制剂。在用CP-24879长期培养的小鼠肥大细胞瘤ABMC-7细胞中,存在与AA耗竭程度和白三烯C4(LTC4)产生减少相关的去饱和酶活性的浓度依赖性抑制。在外源性AA存在的情况下,通过刺激细胞恢复了LTC4的产生,表明内源性AA作为底物是有限的。[1]
|
| 细胞实验 |
Δ5D/Δ6D联合抑制剂CP-24879显著减少了肝细胞内脂质积聚和炎症损伤。有趣的是,CP-24879在脂肪-1和ω-3处理的肝细胞中表现出优异的抗乳糖和抗炎作用。肝细胞与CP-24879(一种特异性Δ5/Δ6脱饱和酶抑制剂)、17 CAY10566(一种选择性Δ9脱饱和酶抑制因子)、18 EPA或resolvin D1(RvD1)一起孵育,详见在线补充材料和方法。[2]
接下来,我们使用了选择性FADS2抑制剂SC-26196和FADS1/FADS2双重抑制剂CP-24879。由于几种抑制剂通常具有内在的抗氧化活性,我们首先测量了FADS抑制剂在无细胞条件下对2,2-二苯基-1-苦肼基(DPPH)的清除能力。与先前报告的结果类似,在我们的实验条件下,铁他汀-1在10至50μM的浓度下显示出自由基清除活性。虽然SC-26196没有显示出抗氧化潜力,但高浓度的CP-24879在30分钟内清除了60%的DPPH自由基(SI附录,图S5)。为了排除CP-24879的抗氧化作用,在后续实验中使用了低浓度(5μM)的抑制剂,但没有体外抗氧化活性。SC-26196或CP-24879处理对去饱和酶活性的抑制显著降低了RSL3诱导的细胞毒性(图4A和B)。此外,在SC-26196或CP-24879存在的情况下,RSL3诱导的脂质过氧化明显减少(图4C)。接下来,我们评估了在谷胱甘肽耗竭条件下,多不饱和脂肪酸生物合成途径是否也是铁下垂所必需的。首先,在ELOVL5或FADS1耗竭的细胞中,半胱氨酸/蛋氨酸剥夺诱导的铁下垂得到了改善(图4D)。此外,SC-26196或CP-24879在半胱氨酸/蛋氨酸剥夺条件下抑制了细胞死亡(图4E)。基于这些数据,PUFA合成酶在脂质过氧化和铁中毒中起着至关重要的作用。[3] |
| 动物实验 |
In the livers of mice treated chronically with the maximally tolerated dose of CP-24879 (3 mg/kg, t.i.d.), combined delta5/delta6 desaturase activities were inhibited approximately 80% and AA was depleted nearly 50%. These results suggest that delta5 and/or delta6 desaturase inhibitors have the potential to manifest an anti-inflammatory response by decreasing the level of AA and the ensuing production of eicosanoids.[1]
|
| 参考文献 |
|
| 其他信息 |
The anti-inflammatory properties of essential fatty acid deficiency or n-3 polyunsaturated fatty acid supplementation have been attributed to a reduced content of arachidonic acid (AA; 20:4 n-6). An alternative, logical approach to depleting AA would be to decrease endogenous synthesis of AA by selectively inhibiting the delta5 and/or the delta6 fatty acid desaturase. High-throughput radioassays were developed for quantifying delta5, delta6, and delta9 desaturase activities in vitro and in vivo. CP-24879 (p-isopentoxyaniline), an aniline derivative, was identified as a mixed delta5/delta6 desaturase inhibitor during the screening of chemical and natural product libraries. In mouse mastocytoma ABMC-7 cells cultured chronically with CP-24879, there was a concentration-dependent inhibition of desaturase activity that correlated with the degree of depletion of AA and decreased production of leukotriene C4 (LTC4). Production of LTC4 was restored by stimulating the cells in the presence of exogenous AA, indicating that endogenous AA was limiting as substrate. In the livers of mice treated chronically with the maximally tolerated dose of CP-24879 (3 mg/kg, t.i.d.), combined delta5/delta6 desaturase activities were inhibited approximately 80% and AA was depleted nearly 50%. These results suggest that delta5 and/or delta6 desaturase inhibitors have the potential to manifest an anti-inflammatory response by decreasing the level of AA and the ensuing production of eicosanoids.[1]
Using oligonucleotide microarray analysis we identified a significant enrichment of genes involved in the multi-step catalysis of long-chain polyunsaturated fatty acids, namely, Δ-5 desaturase (Δ5D) and Δ6D in NASH. Increased expression of Δ5D and Δ6D at both mRNA and protein level were confirmed in livers from mice with high-fat diet-induced obesity and NASH. Gas chromatography analysis revealed impaired desaturation fluxes toward the ω-6 and ω-3 pathways resulting in increased ω-6 to ω-3 ratio and reduced ω-3 index in human and mouse fatty livers. Restoration of hepatic ω-3 content in transgenic fat-1 mice expressing an ω-3 desaturase, which allows the endogenous conversion of ω-6 into ω-3 fatty acids, produced a significant reduction in hepatic insulin resistance, steatosis, macrophage infiltration, necroinflammation and lipid peroxidation, accompanied by attenuated expression of genes involved in inflammation, fatty acid uptake and lipogenesis. These results were mostly reproduced by feeding obese mice with an exogenous ω-3-enriched diet. A combined Δ5D/Δ6D inhibitor, CP-24879, significantly reduced intracellular lipid accumulation and inflammatory injury in hepatocytes. Interestingly, CP-24879 exhibited superior antisteatotic and anti-inflammatory actions in fat-1 and ω-3-treated hepatocytes. Conclusions: These findings indicate that impaired hepatic fatty acid desaturation and unbalanced ω-6 to ω-3 ratio play a role in the pathogenesis of NASH.[2] Ferroptosis is an iron-dependent regulated necrosis mediated by lipid peroxidation. Cancer cells survive under metabolic stress conditions by altering lipid metabolism, which may alter their sensitivity to ferroptosis. However, the association between lipid metabolism and ferroptosis is not completely understood. In this study, we found that the expression of elongation of very long-chain fatty acid protein 5 (ELOVL5) and fatty acid desaturase 1 (FADS1) is up-regulated in mesenchymal-type gastric cancer cells (GCs), leading to ferroptosis sensitization. In contrast, these enzymes are silenced by DNA methylation in intestinal-type GCs, rendering cells resistant to ferroptosis. Lipid profiling and isotope tracing analyses revealed that intestinal-type GCs are unable to generate arachidonic acid (AA) and adrenic acid (AdA) from linoleic acid. AA supplementation of intestinal-type GCs restores their sensitivity to ferroptosis. Based on these data, the polyunsaturated fatty acid (PUFA) biosynthesis pathway plays an essential role in ferroptosis; thus, this pathway potentially represents a marker for predicting the efficacy of ferroptosis-mediated cancer therapy.[3] |
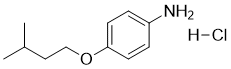
| 分子式 |
C11H18CLNO
|
|---|---|
| 分子量 |
215.721
|
| 精确质量 |
215.107
|
| 元素分析 |
C, 61.25; H, 8.41; Cl, 16.43; N, 6.49; O, 7.42
|
| CAS号 |
10141-51-2
|
| 相关CAS号 |
10141-51-2
|
| PubChem CID |
16078965
|
| 外观&性状 |
Brown to black solid powder
|
| 沸点 |
322.1ºC at 760 mmHg
|
| 熔点 |
154-159ºC
|
| 闪点 |
148.6ºC
|
| 蒸汽压 |
0.000208mmHg at 25°C
|
| LogP |
4.076
|
| tPSA |
35.25
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
14
|
| 分子复杂度/Complexity |
128
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC(C)CCOC1=CC=C(C=C1)N.Cl
|
| InChi Key |
GFESZSNFRSACMU-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C11H17NO.ClH/c1-9(2)7-8-13-11-5-3-10(12)4-6-11;/h3-6,9H,7-8,12H2,1-2H3;1H
|
| 化学名 |
4-(3-methylbutoxy)aniline;hydrochloride
|
| 别名 |
CP 24879 hydrochloride; CP24879 hydrochloride; CP-24879 hydrochloride; CP 24,879 (hydrochloride); CP-24879 (hydrochloride); 4-(3-methylbutoxy)aniline hydrochloride; Benzenamine, 4-(3-methylbutoxy)-, hydrochloride (9CI); p-(Isoamyloxy)aniline hydrochloride; p-(Isopentyloxy)-aniline; . CP-24879 hydrochloride
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~43 mg/mL (~199.3 mM)
Ethanol: ~43 mg/mL (~199.3 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (11.59 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (9.64 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.6356 mL | 23.1782 mL | 46.3564 mL | |
| 5 mM | 0.9271 mL | 4.6356 mL | 9.2713 mL | |
| 10 mM | 0.4636 mL | 2.3178 mL | 4.6356 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|