| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
HCV genotype 1b N(IC50=2.2 ± 0.3 nM);HCV genotype 1a H77(IC50=2.8 ± 0.2 nM);HCV genotype 1b BK(IC50=3.1 ± 0.21 nM);HCV genotype 1b Con1(IC50=0.7 ± 1.4 nM)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:Dasabuvir 对 HCV 基因型 1 聚合酶的抑制选择性至少是人类/哺乳动物聚合酶的 7,000 倍。 Dasabuvir 抑制基因型 1 实验室菌株酶(H77、BK、N 和 Con1 菌株)以及 HCV 基因型 1 感染受试者聚合酶基因产生的酶的聚合酶活性,IC50 介于 2.2 至 10.7 nM 之间。 Dasabuvir 在细胞培养测定中抑制 HCV 亚基因组复制子的复制,针对基因型 1a (H77) 和 1b (Con1) 的 EC50 值分别为 7.7 和 1.8 nM。在 40% 人血浆存在的情况下,抑制效力下降 12 至 13 倍,HCV 基因型 1a (H77) 和 1b (Con1) 复制子的 EC50 分别为 99 和 21 nM。激酶测定:Replizyme Ltd.(英国赫斯灵顿)评估了人类和哺乳动物 DNA 聚合酶的抑制作用。使用 HeLa 细胞提取物中存在的聚合酶和含有聚合酶 II 或聚合酶 III 特异性启动子的 DNA 模板测量人 RNA 聚合酶 II 和 III 的 DNA 依赖性 RNA 聚合酶活性。 α-鹅膏蕈碱是一种强效的人聚合酶 II 抑制剂,也是一种适度的聚合酶 III 抑制剂,被用作对照。反应混合物含有 20 mM Tris-HCl (pH 8.0)、20% 甘油、100 mM KCl、1 mM 二硫苏糖醇 (DTT)、0.2 mM EDTA、6 mM MgCl2 和 1 μg/μl pAdVAntage 质粒(Promega,Madison, WI)(用于聚合酶 III 测定)或 25 ng/μl 巨细胞病毒 (CMV) 启动子 DNA(Promega,麦迪逊,WI)(用于聚合酶 II 测定)。反应混合物还含有 HeLa 细胞核提取物(ProteinOne,Rockville,MD)、400 μM ATP、CTP 和 UTP,以及 16 μM GTP 和 [α-33P]GTP。将反应混合物在 30°C 下孵育 1 小时,加入蛋白酶 K、SDS 和 EDTA 淬灭,在 56°C 下孵育 30 分钟,然后在 Criterion Bio-Rad 5% 丙烯酰胺 Tris-硼酸盐上进行分析-EDTA (TBE)-尿素凝胶。将凝胶干燥并置于 PhosphorImager 屏幕上过夜曝光。测量产物条带的体积,并计算抑制百分比;使用以下公式计算 IC50 值:抑制百分比 = 100[I]/([I] + IC50)。细胞测定:Dasabuvir (ABT-333) 对 HCV 基因型 1 聚合酶的抑制选择性至少是人类/哺乳动物聚合酶的 7,000 倍。 Dasabuvir (ABT-333) 抑制基因型 1 实验室菌株酶(H77、BK、N 和 Con1 菌株)以及由 HCV 基因型 1 感染受试者的聚合酶基因产生的酶的聚合酶活性,IC50 介于 2.2 和10.7纳米。 Dasabuvir (ABT-333) 在细胞培养测定中抑制 HCV 亚基因组复制子的复制,针对基因型 1a (H77) 和 1b (Con1) 的 EC50 值分别为 7.7 和 1.8 nM。在 40% 人血浆存在的情况下,抑制效力下降 12 至 13 倍,HCV 基因型 1a (H77) 和 1b (Con1) 复制子的 EC50 分别为 99 和 21 nM。
|
||
| 酶活实验 |
Replizyme Ltd.(英国赫斯灵顿)评估了对人类和哺乳动物 DNA 聚合酶的抑制作用。使用 HeLa 细胞提取物中存在的聚合酶和含有聚合酶 II 或聚合酶 III 特异性启动子的 DNA 模板测量人 RNA 聚合酶 II 和 III 的 DNA 依赖性 RNA 聚合酶活性。 α-鹅膏蕈碱是一种强效的人聚合酶 II 抑制剂,也是一种适度的聚合酶 III 抑制剂,被用作对照。反应混合物含有 20 mM Tris-HCl (pH 8.0)、20% 甘油、100 mM KCl、1 mM 二硫苏糖醇 (DTT)、0.2 mM EDTA、6 mM MgCl2 和 1 μg/μl pAdVAntage 质粒(Promega,Madison, WI)(用于聚合酶 III 测定)或 25 ng/μl 巨细胞病毒 (CMV) 启动子 DNA(Promega,麦迪逊,WI)(用于聚合酶 II 测定)。反应混合物还含有 HeLa 细胞核提取物(ProteinOne,Rockville,MD)、400 μM ATP、CTP 和 UTP,以及 16 μM GTP 和 [α-33P]GTP。将反应混合物在 30°C 下孵育 1 小时,加入蛋白酶 K、SDS 和 EDTA 淬灭,在 56°C 下孵育 30 分钟,然后在 Criterion Bio-Rad 5% 丙烯酰胺 Tris-硼酸盐上进行分析-EDTA (TBE)-尿素凝胶。将凝胶干燥并置于 PhosphorImager 屏幕上过夜曝光。测量产物条带的体积,并计算抑制百分比;使用以下公式计算 IC50 值:抑制百分比 = 100[I]/([I] + IC50)。
|
||
| 细胞实验 |
Dasabuvir (ABT-333) 对 HCV 基因型 1 聚合酶的抑制选择性至少是人类/哺乳动物聚合酶的 7,000 倍。 Dasabuvir (ABT-333) 抑制基因型 1 实验室菌株酶(H77、BK、N 和 Con1 菌株)以及由 HCV 基因型 1 感染受试者的聚合酶基因产生的酶的聚合酶活性,IC50 介于 2.2 和10.7纳米。 Dasabuvir (ABT-333) 在细胞培养测定中抑制 HCV 亚基因组复制子的复制,针对基因型 1a (H77) 和 1b (Con1) 的 EC50 值分别为 7.7 和 1.8 nM。在 40% 人血浆存在的情况下,抑制效力下降 12 至 13 倍,HCV 基因型 1a (H77) 和 1b (Con1) 复制子的 EC50 分别为 99 和 21 nM。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Dasabuvir reaches peak plasma concentration 4 hours after administration. The absolute bioavailability of Dasabuvir is 70%. Dasabuvir is mainly excreted in the feces (94.4%) with very little excreted in the urine (2%). 26.2% and 0.03% of the drug excreted in the feces and urine respectively was present as the parent compound suggesting metabolism as the major elimination pathway. Dasabuvir has a volume of distribution at steady state of 149 liters. Clearance of Dasabuvir has not been determined. Metabolism / Metabolites Dasabuvir is predominantly metabolized by CYP2C8, and to a lesser extent by CYP3A. Biological Half-Life The half-life of elimination of dasabuvir is 5.5 to 6 hours. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Dasabuvir has not been studied in nursing mothers being treated for hepatitis C infection. Because it is greater than 99.5% bound to maternal plasma proteins, amounts in breastmilk are likely to be very low. If dasabuvir used alone or in combination with sofosbuvir or with ombitasvir, paritaprevir and ritonavir (Viekira Pak) is required by the mother, it is not a reason to discontinue breastfeeding. Some sources recommend against breastfeeding when dasabuvir is used with ribavirin. Ritonavir used as a booster has been studied in several studies of breastfeeding mothers. It is excreted into milk in measurable concentrations and low levels can be found in the blood of some breastfed infants. No reports of adverse reactions in breastfed infants have been reported. For more information, refer to the LactMed record on ritonavir. Hepatitis C is not transmitted through breastmilk and breastmilk has been shown to inactivate hepatitis C virus (HCV). However, the Centers for Disease Control recommends that mothers with HCV infection should consider abstaining from breastfeeding if their nipples are cracked or bleeding. It is not clear if this warning would apply to mothers who are being treated for hepatitis C. Infants born to mothers with HCV infection should be tested for HCV infection; because maternal antibody is present for the first 18 months of life and before the infant mounts an immunologic response, nucleic acid testing is recommended. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Dasabuvir is greater than 99.5% bound to human plasma proteins. |
||
| 参考文献 | |||
| 其他信息 |
Dasabuvir is a member of the class of pyrimidone, which is (as the monohydrate of its sodium salt) in combination with ombitasvir, paritaprevir and ritonavir (under the trade name Viekira Pak) for treatment of chronic hepatitis C virus genotype 1 infection as well as cirrhosis of the liver. It has a role as an antiviral drug and a nonnucleoside hepatitis C virus polymerase inhibitor. It is a member of naphthalenes, a sulfonamide, an aromatic ether and a pyrimidone. It is functionally related to a uracil.
Dasabuvir is a direct acting antiviral medication used as part of combination therapy to treat chronic Hepatitis C, an infectious liver disease caused by infection with Hepatitis C Virus (HCV). HCV is a single-stranded RNA virus that is categorized into nine distinct genotypes, with genotype 1 being the most common in the United States, and affecting 72% of all chronic HCV patients. Treatment options for chronic Hepatitis C have advanced significantly since 2011, with the development of Direct Acting Antivirals (DAAs) such as Dasabuvir. Dasabuvir is a non-nucleoside NS5B inhibitor which binds to the palm domain of NS5B and induces a conformational change which renders the polymerase unable to elongate viral RNA. The binding sites for non-nucleoside NS5B inhibitors are poorly conserved across HCV genotypes leading to the restriction of Dasabuvir's use to genotype 1 only. In a joint recommendation published in 2016, the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) recommend Dasabuvir as first line therapy in combination with [DB09296], [DB09297], and [DB00503] for genotype 1b and with [DB00811] for genotype 1a of Hepatitis C. Dasabuvir, [DB09296], [DB09297], [DB00503], and [DB00811] are used with the intent to cure, or achieve a sustained virologic response (SVR), after 12 weeks of therapy. SVR and eradication of HCV infection is associated with significant long-term health benefits including reduced liver-related damage, improved quality of life, reduced incidence of Hepatocellular Carcinoma, and reduced all-cause mortality. Dasabuvir is available as a fixed dose combination product with [DB09296], [DB09297], and [DB00503] (tradename Viekira Pak) used for the treatment of chronic Hepatitis C. Approved in December 2014 by the FDA, Viekira Pak is indicated for the treatment of HCV genotype 1a with [DB00811] or genotype 1b without [DB00811]. When combined together, Dasabuvir [DB09296], [DB09297], and [DB00503] as the combination product Viekira Pak have been shown to achieve a SVR of 100% for genotype 1b and 89% or 95% for genotype 1a after 12 weeks or 24 weeks of treatment including [DB00811]. Dasabuvir is a Hepatitis C Virus Non-Nucleoside NS5B Palm Polymerase Inhibitor. The mechanism of action of dasabuvir is as a RNA Replicase Inhibitor, and UGT1A1 Inhibitor, and Breast Cancer Resistance Protein Inhibitor. Dasabuvir is a non-nucleoside inhibitor of the hepatitis C virus (HCV) non-structural protein 5B (NS5B), an RNA-dependent RNA polymerase, with potential activity against HCV. Upon administration and after intracellular uptake, dasabuvir binds HCV NS5B polymerase and blocks viral RNA synthesis and replication. The HCV NS5B protein is essential for the replication of the HCV RNA genome. HCV is a small, enveloped, single-stranded RNA virus belonging to the Flaviviridae family; HCV infection is associated with the development of hepatocellular carcinoma (HCC). Drug Indication Dasabuvir, in combination with [DB09296], [DB09297], and [DB00503] (as Viekira Pak) is indicated for the treatment of patients with HCV genotype 1a with [DB00811] or genotype 1b without [DB00811] including those with compensated cirrhosis. FDA Label Exviera is indicated in combination with other medicinal products for the treatment of chronic hepatitis C (CHC) in adults. For hepatitis C virus (HCV) genotype specific activity. Mechanism of Action Dasabuvir is a non-nucleoside inhibitor of the HCV RNA-dependent RNA polymerase encoded by the NS5B gene, which is essential for replication of the viral genome. Based on drug resistance mapping studies of HCV genotypes 1a and 1b, dasabuvir targets the palm domain of the NS5B polymerase, and is therefore referred to as a non-nucleoside NS5B-palm polymerase inhibitor. The EC50 values of dasabuvir against genotype 1a-H77 and 1b-Con1 strains in HCV replicon cell culture assays were 7.7 nM and 1.8 nM, respectively. By binding to NS5b outside of the active site of the enzyme, dasabuvir induces a conformational change thereby preventing further elongation of the nascent viral genome. A limitation of binding outside of the active site is that these binding sites are poorly preserved across the viral genotypes. This results in a limited potential for cross-genotypic activity and increased potential for development of resistance. Dasabuvir is therefore limited to treating genotypes 1a and 1b, and must be used in combination with other antiviral products. |
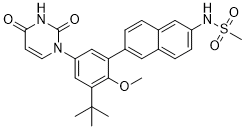
| 分子式 |
C26H27N3O5S
|
|---|---|
| 分子量 |
493.57
|
| 精确质量 |
493.167
|
| 元素分析 |
C, 63.27; H, 5.51; N, 8.51; O, 16.21; S, 6.50
|
| CAS号 |
1132935-63-7
|
| 相关CAS号 |
Dasabuvir sodium;1132940-11-4
|
| PubChem CID |
56640146
|
| 外观&性状 |
White to off-white solid powder.
|
| 密度 |
1.3±0.1 g/cm3
|
| 折射率 |
1.641
|
| LogP |
3.68
|
| tPSA |
118.9
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
35
|
| 分子复杂度/Complexity |
938
|
| 定义原子立体中心数目 |
0
|
| SMILES |
S(C([H])([H])[H])(N([H])C1C([H])=C([H])C2=C(C=1[H])C([H])=C([H])C(=C2[H])C1C([H])=C(C([H])=C(C=1OC([H])([H])[H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H])N1C([H])=C([H])C(N([H])C1=O)=O)(=O)=O
|
| InChi Key |
NBRBXGKOEOGLOI-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C26H27N3O5S/c1-26(2,3)22-15-20(29-11-10-23(30)27-25(29)31)14-21(24(22)34-4)18-7-6-17-13-19(28-35(5,32)33)9-8-16(17)12-18/h6-15,28H,1-5H3,(H,27,30,31)
|
| 化学名 |
N-(6-(3-(tert-butyl)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-2-methoxyphenyl)naphthalen-2-yl)methanesulfonamide
|
| 别名 |
ABT333; ABT-333; ABT 333, Dasabuvir; Trade names: Viekira Pak
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 46~98 mg/mL ( 93.20 ~198.55 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.07 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.07 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.07 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.5 mg/mL (5.07 mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0261 mL | 10.1303 mL | 20.2606 mL | |
| 5 mM | 0.4052 mL | 2.0261 mL | 4.0521 mL | |
| 10 mM | 0.2026 mL | 1.0130 mL | 2.0261 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
PBPK simulations of the pharmacokinetic profiles ofdasabuvirfollowing a single intravenous dose (a,b) or oral dose (c,d) in healthy volunteers.Clin Pharmacol Ther. 2017 Oct; 102(4): 679–687. |
|---|