| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
dBET1 is a proteolysis-targeting chimera (PROTAC) that simultaneously binds bromodomain-containing protein 4 (BRD4, a BET family member) and cereblon (CRBN, a substrate receptor of the CRL4 E3 ubiquitin ligase) (BRD4 BD1: Ki = 0.5 μM for binding [1]
; CRBN: Ki = 1.2 μM for binding [1] ; DC50 (half-maximal degradation concentration) of BRD4 in RS4;11 cells = 10 nM [1] ; >50-fold selectivity for BRD4 over BRD2 (DC50 = 550 nM) and BRD3 (DC50 = 620 nM) [1] ; no significant binding to non-BET bromodomains (e.g., BRD7, BRD9) or other E3 ligase substrates (IC50 > 10 μM) [1] ) |
|---|---|
| 体外研究 (In Vitro) |
dBET1 处理可抑制 MYC 和 PIM1 的转录。当 BRD4 被 dBET1 降解时,MV4;11 细胞系表现出更严重的凋亡效应。 dBET1 治疗后 8 小时,细胞凋亡增加,仅 4 小时后就显着升高。此外,在整个 24 小时期间,dBET1 对 MV4;11 细胞生长表现出强烈且卓越的抑制作用(根据 ATP 含量测定,IC50= 0.14 μM,而 JQ1 的 IC50= 1.1 μM)[1]。
1. dBET1(1-1000 nM)在人急性淋巴细胞白血病(ALL)细胞系RS4;11中剂量依赖性诱导BRD4的泛素化和蛋白酶体降解,DC50为10 nM;50 nM浓度下4小时内实现最大降解(>90%),且效果持续24小时(蛋白质免疫印迹)[1] 2. 在RS4;11和MV4;11(MLL重排白血病)细胞中,dBET1抑制细胞增殖的IC50值分别为3 nM和5 nM(72小时CCK-8实验);在BRD4缺失的细胞系中无显著抗增殖活性(IC50>10 μM)[1] 3. 该化合物(50 nM)在6小时内使RS4;11细胞中关键BRD4靶基因c-Myc的mRNA表达下调85%(qPCR)、蛋白表达下调90%(蛋白质免疫印迹),同时伴随MYC调控基因(CCND1、BCL2)的表达降低60-70%[1] 4. dBET1(25 nM)处理RS4;11细胞24小时和48小时后,分别使凋亡细胞比例增加45%(Annexin V/PI染色)和60%(切割的caspase-3/PARP蛋白质免疫印迹)[1] 5. 在人多发性骨髓瘤细胞系MM.1S中,dBET1(100 nM)使BRD4降解80%,并在克隆形成实验中抑制75%的集落形成;蛋白酶体抑制剂硼替佐米(10 nM)可逆转该效应,证实其依赖蛋白酶体的作用机制[1] 6. 蛋白质组学分析显示,dBET1对脱靶蛋白(如HDACs、CDK4/6)无显著降解作用,证实其对BET家族蛋白的特异性[1] |
| 体内研究 (In Vivo) |
通过使用连续体积测量,死后测量发现,施用 dBET1 降低了肿瘤重量并抑制了肿瘤发展。在第一次或第二次每日 dBET1(50 mg/kg IP)治疗后 4 小时,BRD4 出现急性药效降解。当在切除的肿瘤中使用 dBET1 代替载体对照时,观察到具有统计学意义的 BRD4 不稳定性、MYC 下调和增殖抑制。小鼠对两周的 dBET1 反应有效,体重、血细胞比容、血小板计数或白细胞计数没有明显变化 [1]。
1. 在RS4;11 ALL异种移植模型(雌性NOD/SCID小鼠)中: - 腹腔注射dBET1(5、10、20 mg/kg,每3天1次,连续14天)剂量依赖性抑制肿瘤生长,肿瘤生长抑制(TGI)率分别为60%、85%和95%[1] - 10 mg/kg剂量使8只小鼠中的5只实现肿瘤完全消退,且停药后30天未观察到肿瘤复发[1] 2. dBET1(10 mg/kg腹腔注射)在给药后6小时内使肿瘤组织中BRD4降解>90%(蛋白质免疫印迹),并使肿瘤内c-Myc蛋白水平降低80%(免疫组化)[1] 3. 在MLL重排白血病的患者来源异种移植(PDX)模型中,dBET1(10 mg/kg腹腔注射,每3天1次)使骨髓中白血病母细胞浸润减少75%(流式细胞术),并将中位生存期从载体组的22天延长至45天[1] 4. 在有效剂量(≤20 mg/kg)下,处理小鼠未观察到显著体重下降(<5%)或毒性临床症状(如嗜睡、进食减少)[1] |
| 酶活实验 |
1. BRD4溴结构域结合实验:将重组人BRD4 BD1蛋白与荧光标记的乙酰化组蛋白H4肽(H4K5ac/K8ac)及系列稀释的dBET1(0.1-10 μM)在实验缓冲液(25 mM Tris-HCl、150 mM NaCl,pH 7.4)中25℃孵育60分钟;检测荧光偏振度以量化BRD4-组蛋白结合的抑制程度,从竞争曲线计算Ki值[1]
2. CRBN结合实验:将重组人CRBN蛋白与沙利度胺衍生的荧光探针及dBET1(0.1-10 μM)在结合缓冲液中25℃孵育90分钟;检测HTRF信号(665 nm/620 nm)以评估dBET1对探针的置换作用,证实其与CRBN的直接结合[1] 3. 泛素化实验:将RS4;11细胞裂解液与重组E1、E2(UbcH5b)、CRL4-CRBN复合物、泛素及dBET1(10-100 nM)在37℃孵育90分钟;通过抗BRD4抗体免疫沉淀后,用抗泛素抗体进行蛋白质免疫印迹,检测BRD4的泛素化水平[1] |
| 细胞实验 |
1. BRD4降解蛋白质免疫印迹实验:将RS4;11和MV4;11细胞以1×10⁶个/孔的密度接种于6孔板,用dBET1(1-1000 nM)在37℃、5% CO₂条件下处理2-24小时;制备全细胞裂解液,经SDS-PAGE电泳后,用抗BRD4、BRD2、BRD3和β-肌动蛋白(内参)的抗体进行检测;通过密度计量法量化条带强度,计算蛋白降解的DC50值[1]
2. 白血病细胞增殖实验:将RS4;11、MV4;11和BRD4缺失的K562细胞以5×10³个/孔的密度接种于96孔板,用dBET1(0.001-10 μM)处理72小时;加入CCK-8试剂孵育2小时,在450 nm处检测吸光度,计算细胞活力和抗增殖活性的IC50值[1] 3. 凋亡检测实验:用dBET1(10-100 nM)处理RS4;11细胞24小时和48小时;收集细胞,经Annexin V-FITC和碘化丙啶(PI)染色后,通过流式细胞术量化凋亡细胞(Annexin V+/PI-和Annexin V+/PI+)比例;同时通过蛋白质免疫印迹检测切割的caspase-3和PARP水平,证实凋亡信号激活[1] 4. c-Myc基因表达qPCR实验:用dBET1(10-50 nM)处理RS4;11细胞6小时;提取总RNA并反转录为cDNA;用c-Myc、CCND1、BCL2和GAPDH(管家基因)的引物进行qPCR;采用2^(-ΔΔCt)法计算相对基因表达量[1] 5. 克隆形成实验:将MM.1S细胞以500个/孔的密度接种于6孔板,用dBET1(10-100 nM)在37℃条件下处理14天;用甲醇固定集落,结晶紫染色后在显微镜下计数;计算相对于载体处理对照组的集落形成抑制百分比[1] |
| 动物实验 |
1. RS4;11 xenograft tumor model: Female NOD/SCID mice (6-8 weeks old) were injected subcutaneously with 5×10⁶ RS4;11 cells into the right flank; tumors were allowed to reach 100-150 mm³ before treatment initiation; dBET1 was formulated in 10% DMSO, 40% PEG400, and 50% sterile saline, and administered intraperitoneally at 5, 10, or 20 mg/kg every 3 days for 14 days (volume: 10 mL/kg); tumor volume was measured every 3 days (volume = length × width² / 2), and mice were euthanized at study end for tumor tissue collection [1]
2. PDX leukemia model: NOD/SCID mice were injected intravenously with bone marrow cells from a patient with MLL-rearranged ALL; 7 days post-injection, dBET1 (10 mg/kg IP q3d) or vehicle was administered for 21 days; bone marrow was harvested at study end, and leukemic blast infiltration was quantified by flow cytometry (CD19/CD34 staining); survival was monitored for 60 days [1] 3. Pharmacodynamic sampling protocol: RS4;11 xenograft mice were treated with a single dose of dBET1 (10 mg/kg IP); tumor tissues were harvested at 2, 6, 12, and 24 hours post-dosing; protein lysates were prepared for western blot analysis of BRD4 and c-Myc levels to determine the duration of target degradation [1] |
| 药代性质 (ADME/PK) |
1. In mice, intraperitoneal administration of dBET1 (10 mg/kg) resulted in peak tumor concentrations of 350 nM at 6 hours post-dosing, with a tumor/plasma ratio of 2.1 [1]
|
| 毒性/毒理 (Toxicokinetics/TK) |
1. dBET1 showed no significant cytotoxicity in normal human peripheral blood mononuclear cells (PBMCs) at concentrations up to 1 μM, with cell viability >90% after 72-hour treatment (CCK-8 assay) [1]
2. In NOD/SCID mice treated with dBET1 (20 mg/kg IP q3d for 14 days), no significant changes in serum liver (ALT, AST) or kidney (BUN, creatinine) function markers were observed [1] 3. Histopathological examination of major organs (liver, kidney, spleen, bone marrow) in treated mice revealed no treatment-related lesions or inflammation [1] |
| 参考文献 | |
| 其他信息 |
1. dBET1 is the first proteolysis-targeting chimera (PROTAC) developed to degrade BET family proteins (primarily BRD4) via the ubiquitin-proteasome system (UPS) [1]
2. The mechanism of action of dBET1 involves dual binding: one end interacts with the bromodomain of BRD4, and the other end binds to CRBN (a component of the CRL4 E3 ligase), forming a ternary complex that triggers ubiquitination and proteasomal degradation of BRD4 [1] 3. dBET1 exhibits potent preclinical efficacy in MLL-rearranged leukemia models, a subtype of cancer driven by BRD4-dependent oncogenic transcription [1] 4. As a PROTAC, dBET1 overcomes limitations of traditional BET inhibitors (e.g., acquired resistance, incomplete target inhibition) by inducing irreversible protein degradation rather than reversible enzymatic inhibition [1] 5. dBET1 has not received FDA approval and is a preclinical tool compound for studying PROTAC technology and BET protein biology; it has not been advanced to clinical trials [1] |
| 分子式 |
C38H37CLN8O7S
|
|---|---|
| 分子量 |
785.267785787582
|
| 精确质量 |
784.219
|
| CAS号 |
1799711-21-9
|
| PubChem CID |
91799313
|
| 外观&性状 |
White to light yellow solid powder
|
| 密度 |
1.6±0.1 g/cm3
|
| 折射率 |
1.746
|
| LogP |
1.98
|
| tPSA |
222
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
11
|
| 可旋转键数目(RBC) |
12
|
| 重原子数目 |
55
|
| 分子复杂度/Complexity |
1550
|
| 定义原子立体中心数目 |
1
|
| SMILES |
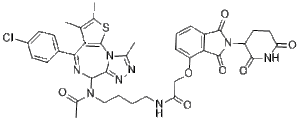
CC1=C(SC2=C1C(=N[C@H](C3=NN=C(N32)C)CC(=O)NCCCCNC(=O)COC4=CC=CC5=C4C(=O)N(C5=O)C6CCC(=O)NC6=O)C7=CC=C(C=C7)Cl)C
|
| InChi Key |
LNKVJWZPPUIOFG-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C38H37ClN8O7S/c1-19-20(2)55-38-30(19)32(23-10-12-24(39)13-11-23)42-33(34-44-43-21(3)46(34)38)45(22(4)48)17-6-5-16-40-29(50)18-54-27-9-7-8-25-31(27)37(53)47(36(25)52)26-14-15-28(49)41-35(26)51/h7-13,26,33H,5-6,14-18H2,1-4H3,(H,40,50)(H,41,49,51)
|
| 化学名 |
N-(4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)-N-(4-(2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)oxy)acetamido)butyl)acetamide
|
| 别名 |
dBET1; d-BET-1; d BET 1; dBET-1; dBET 1; JQ1-Thalidomide conjugate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~43.33 mg/mL (~55.18 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 3 mg/mL (3.82 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 30.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.2734 mL | 6.3672 mL | 12.7345 mL | |
| 5 mM | 0.2547 mL | 1.2734 mL | 2.5469 mL | |
| 10 mM | 0.1273 mL | 0.6367 mL | 1.2734 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。