| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
p300/CBP; E3 ligase Cereblon
|
|---|---|
| 体外研究 (In Vitro) |
在 MM1S 细胞中,dCBP-1(10–1000 nM;6 小时)几乎完全降解 p300/CBP。在其他测试的多发性骨髓瘤细胞系中,例如 MM1R、KMS-12-BM 和 KMS34 细胞,dCBP-1 也可以转导几乎完全的 p300/CBP 降解 [1]。当 dCBP-1 应用于人类单倍体细胞系 HAP1 六小时时,在 10 至 1000 nM 的剂量范围内,CBP 和 p300 几乎完全消失。根据使用 250 nM dCBP-1 的时程分析,治疗一小时后,p300/CBP 几乎完全降解[1]。
|
| 酶活实验 |
dCBP-1消除增强子赖氨酸乙酰化和染色质可及性[1]
p300/CBP乙酰转移酶活性动态调节数千种染色质相关蛋白中的许多赖氨酸残基。组蛋白H3上的赖氨酸27是一种被KAT抑制剂和溴结构域抑制剂抑制的底物,H3K27ac被认为是p300/CBP介导的增强子活性本身所必需的(Raisner等人,2018)。在用A-485、GNE-781、两种抑制剂的组合或dCBP-1处理MM1S细胞6小时后,我们对H3K27ac进行了染色质免疫沉淀测序(ChIP-seq)。单独或联合使用抑制剂对对照细胞中富集位点的H3K27ac水平只有适度的影响,而dCBP-1在这些位点几乎完全丧失了这种修饰(图5A)。这些乙酰化损失区域与p300和CBP高度结合,验证了它们是p300/CBP作用的直接位点。H3K27ac的缺失在p300/CBP结合的致癌IGH增强子上也很明显,该增强子驱动高MYC表达(图5B)。我们还通过ATAC-seq进行了染色质可及性测量,结果显示,仅在dCBP-1处理的细胞中,IGH增强子和MYC启动子的p300/CBP调控位点的可及性染色质显著缺失。这表明,只有通过化学诱导降解最容易实现的从染色质中完全去除p300/CBP,才能完全消除增强子活性,同时减弱与DNA调控元件相关的转录因子。 |
| 细胞实验 |
蛋白质印迹分析[1]
细胞类型: 多发性骨髓瘤细胞系 MM1S 测试浓度: 10 nM、100 nM、250 nM、500 nM、1000 nM 孵育持续时间:6 小时 实验结果:证明快速降解,2 小时后 p300/CBP 几乎完全失去了。 细胞活力测定: 将细胞接种到384孔组织培养处理板中,每孔2000个细胞,在70μL适当的培养基中。每个板的最后一列都接种了不含细胞的培养基。将板在350g下离心以去除气泡,并放置在培养箱中过夜。接种后一天,用35 nL dCBP-1、泊马度胺、A-485或GNE-781处理细胞,用384针一次性复制器将其重新悬浮在DMSO中。将板再次以350g离心,以确保每个孔中的化合物适当混合,并放置在培养箱中。处理四天后,每孔加入7μL alamarBlue(Invitrogen)并孵育24小时。孵育后,在Envision多孔板阅读器上读取单个孔的荧光。通过将信号归一化到DMSO处理的孔来生成剂量反应曲线。使用GraphPad PRISM软件计算AUC值。对于随时间生长的测定,将MM1S细胞以3 x 105个细胞/mL的密度铺在24孔低附着组织培养板上。接种后一天,用每种化合物100nM处理细胞三次,并在五天的处理过程中用血细胞计数器手动计数培养物。 |
| 参考文献 | |
| 其他信息 |
The enhancer factors CREB-binding protein (CBP) and p300 (also known as KAT3A and KAT3B) maintain gene expression programs through lysine acetylation of chromatin and transcriptional regulators and by scaffolding functions mediated by several protein-protein interaction domains. Small molecule inhibitors that target some of these domains have been developed; however, they cannot completely ablate p300/CBP function in cells. Here we describe a chemical degrader of p300/CBP, dCBP-1. Leveraging structures of ligand-bound p300/CBP domains, we use in silico modeling of ternary complex formation with the E3 ubiquitin ligase cereblon to enable degrader design. dCBP-1 is exceptionally potent at killing multiple myeloma cells and can abolish the enhancer that drives MYC oncogene expression. As an efficient degrader of this unique class of acetyltransferases, dCBP-1 is a useful tool alongside domain inhibitors for dissecting the mechanism by which these factors coordinate enhancer activity in normal and diseased cells.[1]
In the studies of dCBP-1 activity in multiple myeloma, researchers do observe augmented effects on gene expression programs, antiproliferation, and chromatin structures when compared with bromodomain and KAT domain inhibitor treatment either alone or in combination. This includes a complete loss of oncogenic enhancer chromatin accessibility and H3K27 acetylation that cannot be achieved with equivalent doses of free inhibitors. While CRBN-based heterobifunctional degraders of BET proteins have similar antiproliferative and anti-MYC effects in cellular models of multiple myeloma, the functional domains outside their bromodomains are largely divergent, and so it is likely that the acute effects of p300/CBP loss on chromatin composition and enhancer structures are distinct (Lim et al., 2019). The addition of dCBP-1 to the toolbox of chromatin regulator-targeting degraders (along with degraders of BET factors, BRD9, TRIM24, SMARCA2/4, CDK9, EED/EZH2, PCAF/GCN5, HDAC1/2/3) will thus enable precise investigation of their unique functions (Bassi et al., 2018; Farnaby et al., 2019; Gechijian et al., 2018; Hsu et al., 2019; Olson et al., 2018; Potjewyd et al., 2019; Remillard et al., 2017; Smalley et al., 2020; Winter et al., 2015). Further studies will also assess the tolerability of p300/CBP degradation in animal models, which will enable additional evaluation of the therapeutic potential of dCBP-1 and other pharmacologically optimized analogues.[1] The chromatin regulators CBP and p300 play important roles in maintaining enhancer-driven gene transcription in normal and malignant cells. Their enzymatic activity through ε-N-lysine acetylation establishes these posttranslational marks on histones, transcription factors, and an array of other chromatin regulators. In addition to their acetyltransferase activity, p300/CBP also possess a number of other functional domains that mediate protein:protein interactions and scaffolding functions at enhancers. Several high-quality selective chemical inhibitors of p300/CBP function have been developed, and efforts to develop them as cancer therapeutics are underway. However, inhibition of single domains alone cannot completely ablate p300/CBP activity in cells. We have discovered a highly potent chemical degrader of both p300 and CBP, dCBP-1, that potently ablates enhancer-mediated transcription. We envision that dCBP-1 will be a useful tool for studying the acute effects of p300/CBP loss and may lead to new therapeutic strategies in cancers such as multiple myeloma that are often driven by oncogenic enhancer activity.[1] |
| 分子式 |
C51H63F2N11O10
|
|---|---|
| 分子量 |
1028.1104
|
| 精确质量 |
1027.472
|
| 元素分析 |
C, 59.58; H, 6.18; F, 3.70; N, 14.99; O, 15.56
|
| CAS号 |
2484739-25-3
|
| PubChem CID |
154690309
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| LogP |
1.6
|
| tPSA |
224Ų
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
16
|
| 可旋转键数目(RBC) |
21
|
| 重原子数目 |
74
|
| 分子复杂度/Complexity |
1960
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
ILVRLRGBSSFKIE-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C51H63F2N11O10/c1-54-51(70)61-17-11-41-40(31-61)47(62-14-3-4-32-26-37(33-29-56-59(2)30-33)38(46(52)53)28-43(32)62)58-64(41)35-9-15-60(16-10-35)45(66)12-18-71-20-22-73-24-25-74-23-21-72-19-13-55-34-5-6-36-39(27-34)50(69)63(49(36)68)42-7-8-44(65)57-48(42)67/h5-6,26-30,35,42,46,55H,3-4,7-25,31H2,1-2H3,(H,54,70)(H,57,65,67)
|
| 化学名 |
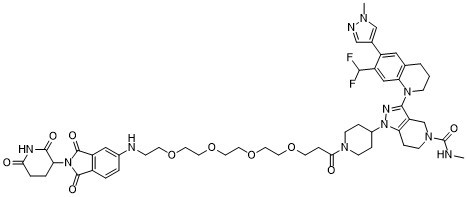
3-[7-(difluoromethyl)-6-(1-methylpyrazol-4-yl)-3,4-dihydro-2H-quinolin-1-yl]-1-[1-[3-[2-[2-[2-[2-[[2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindol-5-yl]amino]ethoxy]ethoxy]ethoxy]ethoxy]propanoyl]piperidin-4-yl]-N-methyl-6,7-dihydro-4H-pyrazolo[4,3-c]pyridine-5-carboxamide
|
| 别名 |
dCBP 1; dCBP-1; CID 154690309; 3-[7-(Difluoromethyl)-6-(1-methylpyrazol-4-yl)-3,4-dihydro-2H-quinolin-1-yl]-1-[1-[3-[2-[2-[2-[2-[[2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindol-5-yl]amino]ethoxy]ethoxy]ethoxy]ethoxy]propanoyl]piperidin-4-yl]-N-methyl-6,7-dihydro-4H-pyrazolo[4,3-c]pyridine-5-carboxamide; SCHEMBL24269061; dCBP1
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~48.63 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 5 mg/mL (4.86 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 50.0mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.86 mg/mL (2.78 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 28.6 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.9727 mL | 4.8633 mL | 9.7266 mL | |
| 5 mM | 0.1945 mL | 0.9727 mL | 1.9453 mL | |
| 10 mM | 0.0973 mL | 0.4863 mL | 0.9727 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。