| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 体内研究 (In Vivo) |
口服给药后,去氧孕烯在大鼠和狗体内被广泛代谢。在大鼠中,去氧孕烯主要在 C3、C5、C11 和 C15 位置代谢。去氧孕烯的 15α-位通过添加羟基进行修饰,然后与葡萄糖醛酸结合。狗主要在 C3 和 C17 位置代谢去氧孕烯[1]。
|
|---|---|
| 动物实验 |
Female Wistar rats, Female beagle dogs
56 μg/kg, 106 mg/kg (Rats); 67 μg/kg, 9.6 mg/kg(Dogs) oral administration |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
After oral administration, desogestrel is rapidly absorbed and it reaches a peak concentration of 2 ng/ml after 1.5 hours. The bioavailability of desogestrel is reported to be in the range of 60-80% and the reported AUC is of 3000 ng.h/ml. Almost all the administered dose is modified to the active metabolite, [etonogestrel]. The elimination of desogestrel is found to be mainly renal corresponding to about 6 times the dose eliminated in the bile. The elimination of desogestrel is only done as the metabolites and not as the unchanged drug and about 85% of the administered dose can be excreted as metabolites after 6-8 days. The apparent volume of distribution of desogestrel is of 1.5 L/kg. The metabolic clearance rate of desogestrel is reported to be of about 2 ml/min/kg. After oral dosing of Cerazette desogestrel (DSG) is rapidly absorbed and converted into etonogestrel (ENG). Under steady-state conditions, peak serum levels are reached 1.8 hours after tablet-intake and the absolute bioavailability of ENG is approximately 70%. In the third cycle of use after a single desogestrel and ethinyl estradiol tablet, maximum concentrations of 3-keto-desogestrel of 2,805 +/- 1,203 pg/mL (mean+/-SD) are reached at 1.4+/-0.8 hours. The area under the curve (AUC) is 33,858+/-11,043 pg/mL (hr) after a single dose. At steady state, attained from at least day 19 onwards, maximum concentrations of 5,840 +/-1,667 pg/mL are reached at 1.4+/-0.9 hours. The minimum plasma levels of 3-keto-desogestrel at steady state are 1,400+/-560 pg/mL. The AUC0-24 at steady state is 52,299+/-17,878 pg/mL (hr). The mean AUC0 for 3-keto-desogestrel at single dose is significantly lower than the mean AUC0-24 at steady state. This indicates that the kinetics of 3-keto-desogestrel are non-linear due to an increase in binding of 3-keto-desogestrel to sex hormone-binding globulin in the cycle, attributed to increased sex hormone-binding globulin levels which are induced by the daily administration of ethinyl estradiol. Sex hormone-binding globulin levels increased significantly in the third treatment cycle from day 1 (150+/-64 nmol/L) to day 21 (230+/-59 nmol/L). Etonogestrel is 95.5-99% bound to serum proteins, predominantly to albumin and to a lesser extent to sex hormone-binding globulin (SHBG). For more Absorption, Distribution and Excretion (Complete) data for DESOGESTREL (8 total), please visit the HSDB record page. Metabolism / Metabolites Desogestrel is rapidly metabolized in the intestinal mucosa and by first-pass hepatic metabolism to form the major metabolite of desogestrel is [etonogestrel] which is the biologically active metabolite. This modification is described by the hydroxylation in C3 of the desogestrel molecule. Later, etonogestrel is metabolized following the normal pathways of steroid metabolism. On the other hand, due to the 11-methylene side chain, desogestrel cannot be metabolized to other progestins. In addition to 3-keto-desogestrel, other phase I metabolites are 3alpha-OH-desogestrel, 3beta-OH-desogestrel, and 3alpha-OH-5alpha-H-desogestrel. These other metabolites are not known to have any pharmacologic effects, and are further converted in part by conjugation (phase II metabolism) into polar metabolites, mainly sulfates and glucuronides. Desogestrel is metabolized via hydroxylation and dehydrogenation to the active metabolite etonogestrel. Etonogestrel is metabolised via sulphate and glucuronide conjugation. Desogestrel is metabolized rapidly and completely in the liver and gut wall. It is metabolized to 3-keto-desogestrel, which mediates its progestogenic effects, and it is not metabolized further to another progestogen. The serum concentrations of 3-keto-desogestrel reached maximum levels within 2-3 hours after oral administration of desogestrel and were subsequently cleared with a half-life of 12-24 hours. The metabolism of desogestrel in microsomes from six hours livers in vitro /were studied/. The main metabolite formed was 3-keto-desogestrel; 3alpha-hydroxydesogestrel and 3beta-hydroxydesogestrel were also detected. The metabolism of desogestrel was inhibited by 50% by primaquine at a concentration of 30 umol/L, but not by levonorgestrel at 250 umol/L. For more Metabolism/Metabolites (Complete) data for DESOGESTREL (7 total), please visit the HSDB record page. Desogestrel has known human metabolites that include 3-beta-hydroxy-desogestrel, Desogestrel 17-O-glucuronide, and 3-alpha-hydroxydesogestrel. Biological Half-Life The terminal half-life of desogestrel is determined to be of 30 hours. Etonogestrel is eliminated with a mean half-life of approximately 30 hours, with no difference between single and multiple dosing. The elimination half-life for 3-keto-desogestrel is approximately 38+/-20 hours at steady state. /3-Keto-desogestrel/ |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Desogestrel is only available in the United States in combination oral contraceptive products containing 150 mcg of desogestrel and 30 mcg of ethinyl estradiol. Based on the available evidence, expert opinion holds that nonhormonal methods are preferred during breastfeeding and progestin-only contraceptive are preferred over combined oral contraceptives in breastfeeding women, especially during the first 4 weeks postpartum. For further information, consult the record entitled, Contraceptives, Oral, Combined. ◉ Effects in Breastfed Infants A nonblinded, nonrandomized study compared oral desogestrel 75 mcg alone daily (n = 42) to an intrauterine device (IUD; n = 40) begun 28 to 56 days postpartum for contraception. No differences in infant length, weight or biparietal head circumferences were found after 1, 4, and 7 treatment cycles. Temporary breast enlargement was reported in 2 infants and increased sweating was reported in 1 infant in the desogestrel group, compared with no adverse effects reported in infants in the IUD group. The growth of some infants were again measured at 1.5 and 2.5 years; no clinically important differences were found. A breastfed (extent not stated) infant developed scrotal hair at 4 months of age. His mother had received the progestin, dydrogestrone, during the first trimester of pregnancy and began taking desogestrel 0.075 mg daily as a contraceptive beginning at 3 months postpartum. His mother discontinued desogestrel after 28 days and the scrotal hair resolved by 11 months of age. Desogestrel was a possible contributing cause of scrotal hair growth in this infant. ◉ Effects on Lactation and Breastmilk A nonblinded, nonrandomized study compared oral desogestrel 75 mcg alone daily (n = 42) to an intrauterine device (n = 40) begun 28 to 56 days postpartum for contraception. During the 7-month trial period, 1 woman dropped out of the trial because of diminished lactation compared with none in the IUD group. At the end of the first and fourth treatment cycle, there were no differences in the amount of milk produced between the desogestrel and IUD groups. No differences in triglyceride, protein or lactose content of milk were found at the end of 1, 4, and 7 cycles of therapy. A nonrandomized study followed 200 women given a desogestrel-only contraceptive 75 mcg daily for 6 months beginning at 6 weeks postpartum and compared them to 200 women who received placebos. No difference was found in the amounts of milk production or infant growth and development between the two groups. In a nonblinded, nonrandomized study in Türkiye of 4964 postpartum women were given the option of desogestrel 75 mcg (Cerazette) as a contraceptive starting at 21 days postpartum. On follow-up, the percentages of women who were breastfeeding at the third, sixth and ninth months postpartum were 68.4%, 54.8% and 58.5%, respectively. The authors concluded that the contraceptive had no negative impact on breastfeeding. Protein Binding The main metabolite of desogestrel is mainly found bound to albumin and sex-hormone binding globulin. Around 96-98% of the administered dose of desogestrel is found bound to plasma proteins from which 40-70% is found bound to sex-hormone binding globulin. |
| 参考文献 | |
| 其他信息 |
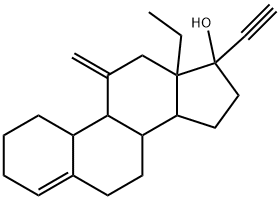
Desogestrel is a 17beta-hydroxy steroid and a terminal acetylenic compound. It has a role as a contraceptive drug, a progestin and a synthetic oral contraceptive.
Desogestrel, a prodrug, is a third generation progestogen and hence, a member of the gonane family which was largely used in Europe before being approved in the US and Canada. It was firstly generated from a study that showed that 11-beta and 11-alkylidene substituent in nortestosterone can enhance the biological activity. Desogestrel is now produced semi-synthetically from naturally occurred plant steroids. In the US, desogestrel is found only in combination with [ethinyl estradiol]. The first approved drug containing desogestrel was developed by Organon USA Inc in 1972 and FDA approved in 1992. Desogestrel is a Progestin. Desogestrel is a synthetic progestogen structurally related to levonorgestrel, with progesterone hormone receptor agonistic activity, used as a contraceptive and hormone replacement agent. Upon administration, desogestrel binds intracellular progesterone receptors in progesterone responsive tissue and the resultant complex interacts with DNA causing either gene transcription or gene repression. This eventually leads to an inhibition of gonadotropin releasing hormone (GnRH) secretion from the hypothalamus and a subsequent inhibition of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) release. This prevents ovulation and alters the cervical mucus. A synthetic progestational hormone used often as the progestogenic component of combined oral contraceptive agents (ORAL CONTRACEPTIVES, COMBINED). Drug Indication Oral desogestrel is used in combination with [ethinylestradiol] as a contraceptive agent for the prevention of pregnancy. Desogestrel is part of the combined oral contraceptives that contain a mix of estrogen and progestin which inhibit ovulation. FDA Label Mechanism of Action Desogestrel enters the cell passively and acts by binding selectively to the progesterone receptor and generating low androgenic activity. Its binding produces an effect like a transcription factor and thus, it produces modifications in the mRNA synthesis. The active metabolite of desogestrel, [etonogestrel], presents a combination of high progestational activity with minimal intrinsic androgenicity. Combination oral contraceptives act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus, which increase the difficulty of sperm entry into the uterus, and changes in the endometrium which reduce the likelihood of implantation. Receptor binding studies, as well as studies in animals, have shown that 3-keto-desogestrel, the biologically active metabolite of desogestrel, combines high progestational activity with minimal intrinsic androgenicity. The relevance of this latter finding in humans is unknown. In contrast to traditional progestogen-only pills, the contraceptive effect of Cerazette is achieved primarily by inhibition of ovulation. Other effects include increased viscosity of the cervical mucus. Recent studies have demonstrated that desogestrel activates the estrogen receptor-alpha at an activity of about 50% of that of 17beta-estradiol but activates the estrogen receptor-beta at an activity of only 20%. Desogestrel and/or its metabolite 3-keto-desogestrel (etonogestrel) were strongly progestogenic (approximately twofold over progesterone), weakly or not androgenic in animal studies in vivo and in-vitro binding assays and weakly or not active on the glucocorticoid receptor. The active metabolite of desogestrel, 3-ketodesogestrel, strongly bound to and activated progesterone receptor-A and, to a slightly lesser extent, progesterone receptor-B Improvement in oral contraceptive formulations was originally achieved through dose reduction of the estrogen and progestogen components. Recently, further improvement was achieved by increasing the selectivity of contraceptive progestins. The ratio between the affinity for the progesterone receptor and the affinity for the androgen receptor is an indicator of progesterone (or androgen) selectivity of a progestin. This ratio (selectivity index) reflects the relative amount of androgenic or progestational effect at a given dose. Relative selectivity can be characterized with in vitro receptor-binding studies and animal pharmacologic experiments. In comparison with levonorgestrel, desogestrel displays markedly lower androgenicity and slightly increased relative progestational activity. In receptor-binding experiments and animal pharmacologic studies, 3-keto-desogestrel, the active metabolite of desogestrel, shows the highest selectivity index. The favorable effect of desogestrel-containing oral contraceptives on lipoprotein metabolism and preexisting androgen-dependent skin disorders and the absence of adverse effects on blood pressure and body weight are attributed to the increased progestin selectivity of desogestrel. For more Mechanism of Action (Complete) data for DESOGESTREL (6 total), please visit the HSDB record page. |
| 分子式 |
C22H30O
|
|---|---|
| 分子量 |
310.47
|
| 精确质量 |
310.229
|
| 元素分析 |
C, 85.11; H, 9.74; O, 5.15
|
| CAS号 |
54024-22-5
|
| 相关CAS号 |
54024-22-5
|
| PubChem CID |
40973
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
428.3±45.0 °C at 760 mmHg
|
| 熔点 |
109-110ºC
|
| 闪点 |
187.9±21.7 °C
|
| 蒸汽压 |
0.0±2.3 mmHg at 25°C
|
| 折射率 |
1.566
|
| LogP |
6.59
|
| tPSA |
20.23
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
23
|
| 分子复杂度/Complexity |
605
|
| 定义原子立体中心数目 |
6
|
| SMILES |
O([H])[C@@]1(C#C[H])C([H])([H])C([H])([H])[C@@]2([H])[C@]3([H])C([H])([H])C([H])([H])C4=C([H])C([H])([H])C([H])([H])C([H])([H])[C@]4([H])[C@@]3([H])C(=C([H])[H])C([H])([H])[C@@]21C([H])([H])C([H])([H])[H]
|
| InChi Key |
RPLCPCMSCLEKRS-BPIQYHPVSA-N
|
| InChi Code |
InChI=1S/C22H30O/c1-4-21-14-15(3)20-17-9-7-6-8-16(17)10-11-18(20)19(21)12-13-22(21,23)5-2/h2,8,17-20,23H,3-4,6-7,9-14H2,1H3/t17-,18-,19-,20+,21-,22-/m0/s1
|
| 化学名 |
(8S,9S,10R,13S,14S,17R)-13-ethyl-17-ethynyl-11-methylidene-1,2,3,6,7,8,9,10,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-ol
|
| 别名 |
Cerazette; DESOGESTREL; Desogen; Org-2969; Desogestrelum
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 16.7~62 mg/mL (53.7~199.7 mM)
Ethanol: ~62 mg/mL (~199.7 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.67 mg/mL (5.38 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 16.7 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1.67 mg/mL (5.38 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 16.7mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1.67 mg/mL (5.38 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.2209 mL | 16.1046 mL | 32.2092 mL | |
| 5 mM | 0.6442 mL | 3.2209 mL | 6.4418 mL | |
| 10 mM | 0.3221 mL | 1.6105 mL | 3.2209 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03895099 | Active Recruiting |
Drug: Desogestrel luteal phase Drug: Desogestrel ovulatory phase |
Infertility | Centre Hospitalier Intercommunal Creteil |
September 4, 2020 | Phase 3 |
| NCT04941833 | Completed | Drug: Desogestrel Oral Tablet | Endometrioma | Rajavithi Hospital | June 1, 2021 | Phase 2 Phase 3 |
| NCT01559480 | Recruiting | Drug: Desogestrel Drug: Placebo |
Endometriosis | Mahidol University | January 2012 | Not Applicable |
| NCT04422028 | Completed | Drug: Desogestrel 0.075 MG | Bioequivalence | Laboratorios Andromaco S.A. | September 16, 2020 | Phase 1 |
| NCT01243697 | Completed | Drug: desogestrel | Ondine Syndrome | Assistance Publique - Hôpitaux de Paris |
April 2011 | Phase 2 Phase 3 |