| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g | |||
| Other Sizes |
| 体内研究 (In Vivo) |
给予大鼠增加剂量的二氟尼柳表明,该药物的药代动力学受到剂量的复杂影响。二氟尼柳的血浆浓度随时间呈指数下降,而当剂量增加时,其半衰期增加。当剂量从 3 mg/kg 增加至 10 mg/kg 时,CLP 急剧下降,随后在 10 mg/kg 至 60 mg/kg 治疗范围内基本保持稳定。已证明二氟尼柳与大鼠血浆蛋白强烈结合且呈浓度依赖性。在 5 至 300 μg/mL 范围内,未结合的二氟尼柳的比例增加了大约 10 倍 [1]。口服给药后,二氟尼柳的活性大约是阿司匹林的 25 倍、格拉芬宁的 3 倍、zomelar 的 2 倍[2]。
妊娠大鼠在妊娠第9天和第10天口服二氟尼柳(250 mg/kg/天)后,胎鼠心室间隔缺损(VSD)的发生率增加(7窝中的8只胎鼠)。[3] 妊娠兔在妊娠第9、10、11天口服二氟尼柳(250 mg/kg/天)后,诱导胎兔出现膈疝(DH)、VSD、中线缺陷(MD;脐膨出),以及小眼畸形和副血管。[3] |
|---|---|
| 动物实验 |
Diflunisal was suspended daily in a 0.5% aqueous methylcellulose solution. [3]
Pregnant rats were administered diflunisal orally by gavage at 250 mg/kg/day (10 ml/kg body weight) on GDs 9 and 10. [3] Pregnant rabbits were administered diflunisal orally by gavage at 250 mg/kg/day (2 ml/kg body weight) on GDs 9, 10, and 11. [3] Fetuses were collected by cesarean section on GD 21 (rats) and GD 29 (rabbits) and examined for external and visceral developmental anomalies. [3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Rapidly and completely absorbed following oral administration, with a bioavailability of 80-90%. Peak plasma concentrations are achieved 2 - 3 hours following oral administration. The drug is excreted in the urine as two soluble glucuronide conjugates accounting for about 90% of the administered dose. Little or no diflunisal is excreted in the feces. Metabolism / Metabolites Hepatic, primarily via glucuronide conjugation (90% of administered dose). Hepatic, primarily via glucuronide conjugation (90% of administered dose). Route of Elimination: The drug is excreted in the urine as two soluble glucuronide conjugates accounting for about 90% of the administered dose. Little or no diflunisal is excreted in the feces. Half Life: 8 to 12 hours Biological Half-Life 8 to 12 hours |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
The precise mechanism of the analgesic and anti-inflammatory actions of diflunisal is not known. Diflunisal is a prostaglandin synthetase inhibitor. In animals, prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain. Since prostaglandins are known to be among the mediators of pain and inflammation, the mode of action of diflunisal may be due to a decrease of prostaglandins in peripheral tissues. Hepatotoxicity Diflunisal therapy is reported to be associated with a low rate of asymptomatic and transient serum aminotransferase elevations, which may resolve even with drug continuation. Marked aminotransferase elevations (>3 fold elevated) occur rarely. Clinically apparent liver injury with jaundice from diflunisal is uncommon; only case reports have been published. The clinical and histologic features of diflunisal hepatotoxicity, however, are distinct and resemble an immunoallergic hepatitis, which is quite different from the liver injury that occurs with aspirin or other salicylates (Case 1). The latency to onset ranges from 1 to 4 weeks and the pattern of enzyme elevations is typically cholestatic, but can also be mixed. Most patients have immunoallergic manifestations such as rash, fever and arthralgias; eosinophilia or atypical lymphocytosis are also common. A history of aspirin allergy has not been reported among cases with allergic reactions to diflunisal. Diflunisal is not a commonly used drug and is not mentioned in large case series on drug induced liver injury or acute liver failure. Likelihood score: C (probable cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation The small amounts of diflunisal in milk do not appear to pose a serious risk to breastfeeding infants. However, a shorter-acting agent having more published information may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding At least 98 to 99% of diflunisal in plasma is bound to proteins. Toxicity Data LD50: 392 mg/kg (Oral, Rat) (A308) LD50: 439 mg/kg (Oral, Mouse) (A308) LD50: 603 mg/kg (Oral, Rabbit) (A308) Diflunisal induced maternal toxicity in rats, including gastrointestinal (GI) toxicity (adhesions in stomach and intestines, distended GI tract, enlarged mesenteric lymph nodes), weight loss, and reduced food consumption. Three maternal deaths occurred in rats. [3] In rabbits, diflunisal caused maternal deaths (4 does died or were euthanized), loose/liquid stools, decreased activity, rapid respiration, reduced body weight, and reduced food consumption. [3] |
| 参考文献 |
|
| 其他信息 |
Diflunisal can cause developmental toxicity and female reproductive toxicity according to state or federal government labeling requirements.
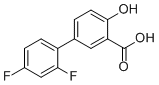
Diflunisal is an organofluorine compound comprising salicylic acid having a 2,4-difluorophenyl group at the 5-position. It has a role as a non-steroidal anti-inflammatory drug and a non-narcotic analgesic. It is an organofluorine compound and a monohydroxybenzoic acid. It is functionally related to a salicylic acid and a 1,3-difluorobenzene. Diflunisal, a salicylate derivative, is a nonsteroidal anti-inflammatory agent (NSAIA) with pharmacologic actions similar to other prototypical NSAIAs. Diflunisal possesses anti-inflammatory, analgesic and antipyretic activity. Though its mechanism of action has not been clearly established, most of its actions appear to be associated with inhibition of prostaglandin synthesis via the arachidonic acid pathway. Diflunisal is used to relieve pain accompanied with inflammation and in the symptomatic treatment of rheumatoid arthritis and osteoarthritis. Diflunisal is a Nonsteroidal Anti-inflammatory Drug. The mechanism of action of diflunisal is as a Cyclooxygenase Inhibitor. Diflunisal is a salicylic acid derivative that is used in the therapy of chronic arthritis and mild to moderate acute pain. Diflunisal has been linked mild, transient elevations in serum aminotransferase levels during therapy as well as to rare instances of idiosyncratic drug induced liver disease. Diflunisal is a difluorophenyl derivate of salicylic acid and a nonsteroidal anti-inflammatory drug (NSAID) with antipyretic, analgesic and anti-inflammatory properties. Diflunisal competitively inhibits both cyclooxygenase (COX) -1 and -2, with higher affinity for COX-1, and subsequently blocks the conversion of arachidonic acid to prostaglandin precursors. This leads to an inhibition of the formation of prostaglandins that are involved in pain, inflammation and fever. Diflunisal differs from other salicylates, in that it is not metabolized to salicylic acid, hence it has a longer half-life. Diflunisal, a salicylate derivative, is a nonsteroidal anti-inflammatory agent (NSAIA) with pharmacologic actions similar to other prototypical NSAIAs. Diflunisal possesses anti-inflammatory, analgesic and antipyretic activity. Though its mechanism of action has not been clearly established, most of its actions appear to be associated with inhibition of prostaglandin synthesis via the arachidonic acid pathway. Diflunisal is used to relieve pain accompanied with inflammation and in the symptomatic treatment of rheumatoid arthritis and osteoarthritis. A salicylate derivative and anti-inflammatory analgesic with actions and side effects similar to those of ASPIRIN. See also: Diflunisal sodium (is active moiety of). Drug Indication For symptomatic treatment of mild to moderate pain accompanied by inflammation (e.g. musculoskeletal trauma, post-dental extraction, post-episiotomy), osteoarthritis, and rheumatoid arthritis. FDA Label Mechanism of Action The precise mechanism of the analgesic and anti-inflammatory actions of diflunisal is not known. Diflunisal is a prostaglandin synthetase inhibitor. In animals, prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain. Since prostaglandins are known to be among the mediators of pain and inflammation, the mode of action of diflunisal may be due to a decrease of prostaglandins in peripheral tissues. Pharmacodynamics Diflunisal is a nonsteroidal drug with analgesic, anti-inflammatory and antipyretic properties. It is a peripherally-acting non-narcotic analgesic drug. Habituation, tolerance and addiction have not been reported. Diflunisal is a difluorophenyl derivative of salicylic acid. Chemically, diflunisal differs from aspirin (acetylsalicylic acid) in two respects. The first of these two is the presence of a difluorophenyl substituent at carbon 1. The second difference is the removal of the 0-acetyl group from the carbon 4 position. Diflunisal is not metabolized to salicylic acid, and the fluorine atoms are not displaced from the difluorophenyl ring structure. Diflunisal is a nonsteroidal anti-inflammatory drug (NSAID) with non-selective COX inhibitory activity. [3] It is associated with fetal developmental defects (VSD, DH, MD) when administered during sensitive windows of heart development and midline closure in rats and rabbits. [3] The study suggests that COX-1 inhibition may be involved in the induction of VSD, while selective COX-2 inhibition appears to have no effect on heart development or midline closure. [3] |
| 分子式 |
C13H8F2O3
|
|---|---|
| 分子量 |
250.1976
|
| 精确质量 |
250.044
|
| CAS号 |
22494-42-4
|
| 相关CAS号 |
Diflunisal-d3;1286107-99-0
|
| PubChem CID |
3059
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
386.9±42.0 °C at 760 mmHg
|
| 熔点 |
32-36 °C
|
| 闪点 |
187.8±27.9 °C
|
| 蒸汽压 |
0.0±0.9 mmHg at 25°C
|
| 折射率 |
1.601
|
| LogP |
4.44
|
| tPSA |
57.53
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
18
|
| 分子复杂度/Complexity |
311
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
HUPFGZXOMWLGNK-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C13H8F2O3/c14-8-2-3-9(11(15)6-8)7-1-4-12(16)10(5-7)13(17)18/h1-6,16H,(H,17,18)
|
| 化学名 |
2',4'-difluoro-4-hydroxy-[1,1'-biphenyl]-3-carboxylic acid
|
| 别名 |
MK-647; MK647; MK 647; trade names: Dolobid; Dolobis; Flovacil; Fluniget; Fluodonil; Dflunisal
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~199.84 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.99 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.99 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.9968 mL | 19.9840 mL | 39.9680 mL | |
| 5 mM | 0.7994 mL | 3.9968 mL | 7.9936 mL | |
| 10 mM | 0.3997 mL | 1.9984 mL | 3.9968 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。