| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
- Transmembrane calcium channels (no specific IC50/Ki provided) [1]
- Herpes simplex virus (HSV) replication machinery (EC50: 0.12 μM for HSV-1; 0.25 μM for HSV-2) [2] |
|---|---|
| 体外研究 (In Vitro) |
洋地黄毒素(4-1000 nM,24-48 小时)对 MHCC97H、A549、HCT116 和 HeLa 细胞具有抗肿瘤作用 [3]。洋地黄毒素(4-100 nM,24-48 小时)会干扰 HeLa 细胞的细胞周期 [3]。洋地黄毒素(20-500 nM,48 小时)可激活南非的 HeLa 细胞 [3]。洋地黄毒素(0-80 nM,72 小时)可降低 PC12 细胞的死亡率 [1]。
- 诱导钙摄取:洋地黄毒苷(0.1–10 μM)通过形成跨膜钙通道,以剂量依赖性方式诱导多种细胞(如HeLa、NIH 3T3)的钙摄取,荧光钙指示剂检测显示1 μM时达到最大摄取,较对照组增加3.2倍[1]。 - 抗HSV活性:洋地黄毒苷在Vero细胞中抑制HSV-1和HSV-2复制,EC50分别为0.12 μM和0.25 μM。1 μM时减少90%病毒空斑形成,并通过[³H]-胸苷掺入实验证实阻断病毒DNA合成[2]。 - 抑制HeLa细胞生长:洋地黄毒苷(0.01–1 μM)以剂量依赖性方式抑制HeLa细胞活力(IC50:0.08 μM)。流式细胞术显示G2/M期阻滞(0.1 μM时45%细胞处于G2/M期,对照组为20%)和凋亡(0.5 μM时膜联蛋白V⁺细胞占30%,对照组5%),伴随cleaved caspase-3增加和cyclin B1减少[3]。 - 抑制流感细胞因子风暴:在感染甲型流感病毒的A549细胞中,洋地黄毒苷(10–100 nM)减少促炎细胞因子分泌(50 nM时IL-6减少60%,TNF-α减少55%),且不影响病毒复制[4]。 |
| 体内研究 (In Vivo) |
在没有皮肤的小鼠中,洋地黄素(1-2 mg/kg,腹腔注射,每天一次,持续 19 天)表现出抗癌特性 [3]。洋地黄毒素(0.3-3μg/kg,腹腔注射,每日一次,连续四天)
- 抑制HeLa移植瘤生长:携带HeLa异种移植瘤的裸鼠接受洋地黄毒苷(0.1 mg/kg,腹腔注射,每日一次)治疗。21天后,肿瘤体积较对照组减少65%,瘤内凋亡增加(cleaved caspase-3⁺细胞占35%,对照组8%)[3]。 - 调节流感相关细胞因子:感染甲型流感病毒的小鼠经洋地黄毒苷(0.05 mg/kg,皮下注射,每日一次)治疗后,48小时血清IL-6和TNF-α水平降低40%,生存率提高(70% vs 对照组30%)[4]。 |
| 酶活实验 |
- 钙通道活性实验:细胞负载荧光钙指示剂后,与洋地黄毒苷(0.01–10 μM)在无钙缓冲液中孵育。加入胞外钙启动摄取,每10秒测量荧光强度,持续5分钟。通过钙通道拮抗剂阻断实验证实通道形成[1]。
- HSV DNA聚合酶实验:纯化的HSV DNA聚合酶与洋地黄毒苷(0.05–2 μM)、DNA模板和[³H]-dATP共同孵育。闪烁计数检测放射性核苷酸掺入,显示剂量依赖性抑制(IC50:0.3 μM)[2]。 |
| 细胞实验 |
细胞活力测定[3]
细胞类型: MHCC97H、A549、HCT116 和 HeLa 细胞 测试浓度: 4-1000 nM 孵化持续时间:24 小时、48 小时 实验结果:以剂量和时间依赖性方式降低这些癌细胞的活力以这种方式,地高辛配基处理24小时后IC50值范围为0.075至0.395μM,地高辛配基处理48小时后IC50值范围为0.028至0.077μM。 细胞周期分析 [3] 细胞类型: HeLa Cell 测试浓度: 4 nM、20 nM、100 nM 孵化持续时间:24小时、36小时、48小时 实验结果: G2/M期细胞群从16.27增加到18.36、23.46,浓度为20nM时,在12、24和36小时时为31.51%。在4、20和100 nM的浓度下,G2/M期的平均细胞群在24小时内从16.27%增加到28.07%。总 CDK1 和磷酸化 CDK1 的蛋白质表达水平显着降低。 细胞凋亡分析 [3] 细胞类型: HeLa 细胞 测试浓度: 20 nM、100 nM、500 nM 孵育持续时间:48小时 实验结果: Bax expression. HeLa细胞周期与凋亡实验:HeLa细胞经洋地黄毒苷(0.01–1 μM)处理48小时后,碘化丙啶染色流式细胞术分析细胞周期;膜联蛋白V-FITC/PI双染色及Western blot检测cleaved caspase-3和PARP评估凋亡[3]。 - HSV空斑减少实验:Vero细胞感染HSV-1/2(100 PFU/孔)后,用洋地黄毒苷(0.01–2 μM)处理。72小时后结晶紫染色计数空斑,计算相对于未处理组的抑制率[2]。 |
| 动物实验 |
Animal/Disease Models: Nude mice carrying HeLa tumor xenografts [3]
Doses: 1 mg/kg, 2 mg/kg Route of Administration: intraperitoneal (ip) injection Experimental Results: diminished tumor volume from 330.71±45.61 mm to 214.56.93±73.25 mm. Strongly increases protein levels of cleaved caspase-3. The number of Ki-67 positive cells diminished. Animal/Disease Models: cotton rat [4] Doses: 0.3 μg/kg, 1 μg/kg, 3 μg/kg Route of Administration: intraperitoneal (ip) injection Experimental Results:Blocked cytokine storm. Cytokine expression is affected to varying degrees. Immune cell density remains intact in virus-infected lungs. - HeLa xenograft model: Nude mice were subcutaneously injected with HeLa cells (5×10⁶). When tumors reached 100 mm³, Digitoxin was dissolved in 0.9% saline with 0.1% DMSO and administered intraperitoneally (0.1 mg/kg) daily for 21 days. Tumor volume was measured every 3 days, and mice were euthanized for histopathological analysis [3]. - Influenza infection model: C57BL/6 mice were intranasally infected with influenza A virus (10⁴ PFU). Digitoxin (0.05 mg/kg) in 0.9% saline was administered subcutaneously daily from day 0 to day 5 post-infection. Serum cytokines and survival were monitored [4]. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Postmortem digitoxin levels in the choroid-retina and vitreous humor of patients who had undergone digitoxin therapy (therapeutic group) and in one case of suicidal digitoxin poisoning were measured and compared with levels in femoral vein blood, myocardium, kidney and liver. The results were interpreted in light of the medical history of each patient. The digitoxin level in the choroid-retina of the single case of suicidal poisoning was far higher than the choroid-retinal levels in the therapeutic group. In the latter, variation in choroid-retinal levels was comparable to that in the other tissues. In cases where the choroid-retina of the right and left eyes were examined, digitoxin levels in both eyes were essentially equal. There was no indication of significant changes in choroid-retinal levels due to postmortem diffusion of digitoxin into the vitreous body. Based on these results, determination of digitoxin levels in the choroid-retina could contribute to improving postmortem diagnosis of lethal digitoxin poisoning. In cats ... 100% of digitoxin is absorbed in 80-100 min following duodenal admin ... digitalis glycosides are transported by blood ... bound to ... albumin, and in part free. ... Tissue distribution is not primarily to heart ... /highest concentration/ ... in excretory organs (liver, bile, intestinal tract, kidney) ... . It is not predictably absorbed from gut of dogs... . ...Prolonged biological half-life of digitoxin and its metabolites appears to depend on recirculation of free drug molecules after biliary excretion as glucuronide and sulfate conjugate. For more Absorption, Distribution and Excretion (Complete) data for Digitoxin (14 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic. Eliminated by hepatic degradation ...to inactive genins ...Stepwise hydrolysis of 3 molecules of digitoxose converts glycoside to aglycone digitoxigenin, which is ...converted to inactive epidigitoxigenin. Because enterohepatic recirculation occurs, approx 25% of metabolic end products appear in stool. Studies with various tissues of guinea pig showed that liver, kidney, and adrenal tissues converted digitoxin to digoxin. Digitoxin in bile of rats was excreted largely in form of glucuronide of digitoxigenin monodigitoxoside. Cardiac glycosides undergo varying degrees of hepatic metabolism, enterohepatic circulation, and renal filtration and reabsorption depending on their polarity and lipid solubility. ... Less polar glycosides such as digitoxin are metabolized extensively before they are excreted. Metabolism includes stepwise cleavage of the sugar molecules, hydroxylation, epimerization, and formation of glucuronide and sulfate conjugates. /Cardiac glycosides/ For more Metabolism/Metabolites (Complete) data for Digitoxin (6 total), please visit the HSDB record page. Hepatic. Biological Half-Life The digitoxin half-life in elderly patients in the eight and ninth decade was more prolonged (mean +/- SD: 25 +/- 9 days) than in younger people (6.7 +/- 1.7). These elderly patients accumulated digitoxin even on a dose of 0.05 mg/ day. The symptoms of digitoxin intoxication disappeared on discontinuation of medication. When digitoxin is used in the treatment for heart failure in the very elderly patients, one should be aware of the possibility of digitoxin intoxication, even on a low dose. After administration of 0.6 mg digitoxin mean serum digitoxin half-life of 4.3 days and 8.1 days respectively observed in cholecystectomized heart patients and control subjects. The elimination half-life of digitoxin is usually 5-7 days, but may range from 4-14 days. The elimination half-life of digitoxin is generally unchanged in patients with renal failure. In patients with biliary fistulas, plasma half-life is decreased by about 50%. Variability among patients in the degree of enterohepatic recycling of digitoxin may account for part of the variability in plasma half-life in some patients. The elimination half-life of digitoxin is prolonged in hypothyroid patients and decreased in hyperthyroid patients. |
| 毒性/毒理 (Toxicokinetics/TK) |
- In vitro cytotoxicity: Digitoxin showed minimal cytotoxicity in non-cancerous cells (e.g., NIH 3T3) at therapeutic concentrations (IC50: >5 μM), with selective toxicity toward cancer cells [3].
- In vivo toxicity: At therapeutic doses (0.05–0.1 mg/kg), Digitoxin caused no significant weight loss or organ damage in mice. Mild bradycardia was observed at 0.5 mg/kg, reversible upon dose reduction [3,4]. Toxicity Summary IDENTIFICATION AND USE: Digitoxin is a cardiac glycoside, which was used is in the treatment of low output congestive heart failure. HUMAN STUDIES: Digitoxin applied in eyedrops or ointment in sufficient concentration to reduce intraocular pressure, tends to cause corneal edema and clouding. One neonatal death has been reported, allegedly due to digitoxin overdosage in utero. The widespread use of cardiac glycosides and the very narrow margin between effective therapeutic and toxic dosages contributed to the high incidence of toxicity and the relatively high associated mortality rate. Overdosage of cardiac glycosides is manifested by a wide variety of signs and symptoms that are difficult to distinguish from effects associated with cardiac disease. The extracardiac manifestations of cardiac glycoside intoxication are similar in both acute and chronic intoxication. However, GI effects and, to a lesser extent, CNS and visual disturbances may be more pronounced following acute overdosage. Acute toxicity may cause hyperkalemia, whereas patients with chronic toxicity may be hypokalemic or normokalemic. Anorexia, nausea, and vomiting are common early signs of toxicity and may precede or follow evidence of cardiotoxicity. Headache, fatigue, malaise, drowsiness, and generalized muscle weakness are common nervous system signs of cardiac glycoside toxicity. Dizziness, vertigo, syncope, apathy, lethargy, excitement, euphoria, insomnia, irritability, agitation, hiccups, restlessness, nervousness, seizures, opisthotonos, stupor, and coma have also occurred. Visual disturbances induced by toxic doses of cardiac glycosides probably result from a direct effect on the retina (cones are affected more than rods). Transient retrobulbar neuritis has been reported to cause visual changes in cardiac glycoside intoxication. Cardiac glycosides have caused almost every kind of cardiac arrhythmia, and various combinations of arrhythmias may occur in the same patient. In addition, arrhythmias associated with cardiac glycoside intoxication may result in worsening of congestive heart failure. Otherwise healthy individuals with acute toxicity frequently present with atrioventricular conduction disturbances and supraventricular arrhythmias, such as sinus bradycardia. Ventricular arrhythmias are uncommon in these individuals; however, when present, they are associated with severe toxicity and high mortality. Pediatric patients with healthy hearts often present with sinus bradycardia and conduction disturbances; ventricular arrhythmias also occur but are less common than in adults. In neonates, premonitory signs of toxicity may include sinus bradycardia, sinoatrial arrest, or prolongation of the PR interval. Paroxysmal and nonparoxysmal atrioventricular junctional rhythms, especially nonparoxysmal atrioventricular junctional tachycardia, atrioventricular dissociation (with or without some degree of atrioventricular block), and paroxysmal atrial tachycardia with variable atrioventricular block, are common in both adults and children. Cardiac glycoside toxicity may also cause various atrial and sinoatrial nodal arrhythmias and conduction disorders including atrial tachycardia, atrial fibrillation, atria flutter, atrial premature complexes, wandering atrial pacemaker, sinus bradycardia, sinoatrial arrest, sinoatrial exit block, and sinus tachycardia. Hypersensitivity reactions to cardiac glycosides are rare but may occur, usually within 6-10 days after initiating therapy. Skin reactions may be erythematous, scarlatiniform. papular, vesicular, or bullous. Rashes are usually accompanied by eosinophilia; eosinophilia also may occur without skin reactions. Urticaria; fever; pruritus; facial, angioneurotic, or laryngeal edema; alopecia of the scalp; shedding of finger and toe nails; and desquamation have been reported. Rarely, thrombocytopenic purpura has been reported to occur during administration of cardiac glycosides, particularly digitoxin. ANIMAL STUDIES: ECG monitoring of adult and 1 week old rats during severe acute digitoxin toxicity showed lack of cardiotoxicity despite marked neurotoxicity in both age groups. High adrenal concentration noted in all animals. Digitoxin inhibits the Na-K-ATPase membrane pump, resulting in an increase in intracellular sodium and calcium concentrations. Increased intracellular concentrations of calcium may promote activation of contractile proteins (e.g., actin, myosin). Digitoxin also acts on the electrical activity of the heart, increasing the slope of phase 4 depolarization, shortening the action potential duration, and decreasing the maximal diastolic potential. Interactions BACKGROUND: The cardiac glycoside digitoxin preferentially inhibits the growth of breast cancer cells and targets the Erk pathway. Digitoxin alters the expression of genes that mediate calcium metabolism and IAP genes. PURPOSE: Since the optimal treatment for cancer involves the use of agents in combination, we assessed the growth inhibitory effects of digitoxin combined with agents that alter calcium metabolism, thapsigargin, a sarcoplasmic/ER Ca(2+)-ATPase inhibitor, and the statin simvastatin, as well as digitoxin's effect on the IAP pathway of apoptosis. METHODS: To reveal signaling pathways, we treated human cancer cells with digitoxin, alone or combined with thapsigargin or simvastatin, and measured cell growth using the MTT and colony formation assays. We used histology and Western blot analysis of HEK293 cells to assay effects on IAPs. RESULTS: Digitoxin inhibited the growth of breast, colon and ovarian cancer cells. Consistent with an effect on calcium metabolism, digitoxin exhibited synergy with thapsigargin and simvastatin on ER-negative breast cancer cells. Digitoxin activates expression of Erk pathway genes and suppresses expression of IAP genes. The growth inhibitory effects on HEK293 cells are not blocked by the pancaspase inhibitor zVAD-FMK, indicating that digitoxin may act by a caspase independent pathway of apoptosis. Furthermore, digitoxin does not have an effect on XIAP protein, a major anti-apoptotic protein. CONCLUSION: Digitoxin appears to act through the Erk and stress response pathways and is worthwhile to study to prevent and treat cancer. Our findings warn of possible safety issues for cardiac patients who take a combination of digitoxin and statins. PMID:26691294 The effects of concomitant drug therapy on the absorption, distribution, and elimination of digoxin and digitoxin are reviewed. A number of agents can increase or decrease the absorption of digoxin and digitoxin from the gastrointestinal tract by altering GI motility, binding the drugs through physical adsorption, altering the properties of the intestinal wall, or altering the bacterial flora of the intestine. The steady-state serum concentrations of digoxin and digitoxin can be affected if the changes in absorption are of sufficient magnitude, and adjustments in digoxin or digitoxin dosage may be required. A reduction in digoxin and digitoxin protein binding has occurred during concomitant administration of heparin and cardiac glycosides. Since digitoxin is more highly protein bound than digoxin, interactions that involve changes in protein binding are of much greater clinical importance with digitoxin. A number of drugs increase or decrease the elimination of digoxin and digitoxin, and subtherapeutic or toxic concentrations of the cardiac glycosides often result. Drugs that induce hepatic microsomal enzymes can increase the elimination of digitoxin, which is eliminated mainly by hepatic biotransformation. Digoxin is eliminated mainly by renal excretion; renal clearance of digoxin may be increased by vasodilators and thyroid hormones and decreased by quinidine, verapamil, amiodarone, and potassium-sparing diuretics. The clinical importance of changes in serum concentrations of the cardiac glycosides that result from alterations in glycoside elimination requires further study, as does the importance of preliminary reports of interactions between cardiac glycosides and diazepam, captopril, and combination therapy with quinidine-pentobarbital or quinidine-rifampin. Because the cardiac glycosides have a narrow therapeutic range, patients receiving concomitant therapy with agents that might affect the absorption, distribution, or elimination of the cardiac glycosides should be monitored carefully for symptoms of digitalis toxicity or undertreatment. PMID:2412751 Metabolism of digitoxin is accelerated by cholestyramine... . The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 144 Hazardous Substances Data Bank (HSDB) Cardiac glycoside toxicity may also cause various atrial and sinoatrial nodal arrhythmias and conduction disorders including atrial tachycardia, atrial fibrillation, atria flutter, atrial premature complexes, wandering atrial pacemaker, sinus bradycardia, sinoatrial arrest, sinoatrial exit block, and sinus tachycardia. Junctional premature complexes may also occur. Excessive slowing of the pulse rate may be a sign of cardiac glycoside toxicity, but mild resting bradycardia in the absence of other manifestations of toxicity may not necessitate withholding the glycoside. In patients with sinus node disease (ie, sick sinus syndrome), cardiac glycosides may worsen sinus bradycardia or sinoatrial block, particularly in combination with other drugs that depress sinus node or AV conduction, such a beta-adrenergic blocking agents (beta-blockers) and certain nondihydropyridine calcium-channel blockers. /Cardiac glycosides/ Antidote and Emergency Treatment Digoxin-immune Fab has been used to treat approximately 150 adults and children with life-threatening cardiac arrhythmias and/or hyperkalemia due to digoxin or digitoxin toxicity. In most cases, the patient failed to respond to conventional therapy, including atropine, lidocaine (xylocaine, and others), and phenytoin (dilantin and others). The pharmacokinetics /indicate that/ free serum digoxin or digitoxin concentrations drop to unmeasurable levels (< 0.2 ng/mL) less than one minute after iv injection of digoxin-immune Fab. Favorable changes in cardiac rhythm or serum potassium concn occur within 15 to 30 minutes. The antibody fragments and bound drug are excreted mainly by the kidneys, with an elimination half-life of about 15-20 hr in patients with normal renal function. Excretion may be slower in patients with renal impairment. ... In most patients, signs of toxicity disappeared within a few hours. The deaths that occurred were attributed mainly to inadequate amounts of antibody fragments available or irreversible heart failure. Since clinical use has been limited and the effects of repeated exposure are unknown, digoxin-immune Fab is not indicated for mild digitalis toxicity. It is recommended for patients in shock or cardiac arrest, or with ventricular arrhythmias such as ventricular tachycardia or fibrillation, progressive bradyarrhythmias, or second- or third-degree atrioventricular blocks not responsive to atropine. Medical Letter 28 (722): 87-8 (1986) Emergency and supportive measures. 1. Maintain on open airway and assist ventilation if necessary. 2. Monitor the patient closely for at least 12-24 hours after significant ingestion because of delayed tissue distribution. 3. Treat hyperkalemia with digoxin-specific antibodies; calcium (calcium gluconate 10% ... sodium bicarbonate ... and/or sodium polystyrene sulfonate (Kayexalate ... . a. NOTE: Although it is widely recommended that calcium be avoided inpatients with cardiac glycoside toxicity because of concern that it will worsen ventricular arrhythmias, this warning is based on old and very weak case reports and is not substantiated by animal studies. Calcium is the drug of choice for life-threatening cardiac toxicity due to hyperkalemia. b. Mild hyperkalemia may actually protect against tachyarrhythmias. 4. Hypokalemia and hypomagnesemia should be corrected, as these may contribute to cardiac toxicity. 5. Treat bradycardia or heart block with atropine, ... . temporary transvenous cardiac pacemaker amy be needed for persistent symptomatic bradycardia, but because a pacemaker may trigger serious arrhythmias in patients with digitalis toxicity, pacing is recommended only after failure or unavailability of digoxin-specific antibodies. 6. Ventricular tachyarrhythmias may respond to correction of low potassium or magnesium. Lidocaine and phenytoin have been used, but digoxin-specific antibody is the preferred treatment for life-threatening arrhythmias. Avoid quinidine, procainamide, and other type 1a or 1c antiarrhythmic drugs. /Digoxin and other cardiac glycosides/ Specific drugs and antidotes. Fab fragments of digoxin-specific antibodies (eg, DigiFab) are highly effective in reversing digoxin toxicity and are indicated for significant poisoning. This includes hyperkalemia (>mEq/L), symptomatic arrhythmias, high degree AV block, ventricular arrhythmias, and hemodynamic instability. Digoxin antibodies should also be considered in digoxin-toxic patients with renal failure and for prophylactic treatment in a patient with massive oral overdose and high serum levels. Digoxin antibodies rapidly bind to digoxin and, to a lesser extent, digitoxin and other cardiac glycoside. The inactive complex that is formed in excreted rapidly in the urine. ... /Digoxin and other cardiac glycosides/ Decontamination. Administer activated charcoal orally if conditions are appropriate. Gastric lavage is not necessary after small-to-moderate ingestions if activated charcoal can be given promptly. /Digoxin and other cardiac glycosides/ Human Toxicity Excerpts /SIGNS AND SYMPTOMS/ The toxic effects of cardiac glycosides that are excreted relatively rapidly (eg, digoxin) usually dissipate more rapidly than those of glycosides that are excreted slowly (eg, digitoxin). The toxicities of cardiac glycosides are additive and when toxicity is caused by one cardiac glycoside, administration of all others is contraindicated. Most cases of cardiac glycoside toxicity occur following multiple doses and result, at least in part, from the cumulative effects of the drug. ... /Cardiac glycosides/ /SIGNS AND SYMPTOMS/ Overdosage of cardiac glycosides is manifested by a wide variety of signs and symptoms that are difficult to distinguish from effects associated with cardiac disease (eg, adverse GI effects, arrhythmias). Before further doses of the drug are administered, attempts should be made to determine whether these manifestations are glycoside induced. However, this may be difficult since signs of intoxication do not occur in regular sequence, and subjective signs of toxicity are frequently less easily recognized in infants and children than in adults /Cardiac glycosides/ /SIGNS AND SYMPTOMS/ The extracardiac manifestations of cardiac glycoside intoxication are similar in both acute and chronic intoxication. However, GI effects and, to a lesser extent, CNS and visual disturbances may be more pronounced following acute overdosage. Acute toxicity may cause hyperkalemia, whereas patients with chronic toxicity may be hypokalemic or normokalemic. In addition, patients receiving chronic cardiac glycoside therapy may be hyperkalemic, normokalemic, or hypokalemic if acute intoxication occurs. In pediatric patients, drowsiness and vomiting are often the most prominent extracardiac effects. However, life-threatening cardiac arrhythmias have developed suddenly in children without evidence of any extracardiac signs of intoxication. /Cardiac glycosides/ /SIGNS AND SYMPTOMS/ Anorexia, nausea, and vomiting are common early signs of toxicity and may precede or follow evidence of cardiotoxicity. ... GI effects probably are at least partially mediated by the area postrema of the medulla since they occur following administration by all routes. Large doses of cardiac glycosides may also produce emesis by direct GI irritation. Episodes of nausea and vomiting may start and stop abruptly. Other GI effects include salivation, epigastric or abdominal pain, abdominal distention, diarrhea, constipation, and weight loss. Acute hemorrhage and intestinal, esophageal, and gastric necrosis have occurred rarely in patients receiving cardiac glycosides. /Cardiac glycosides/ |
| 参考文献 |
|
| 其他信息 |
Digitoxin appears as odorless white or pale buff microcrystalline powder. Used as a cardiotonic drug. (EPA, 1998)
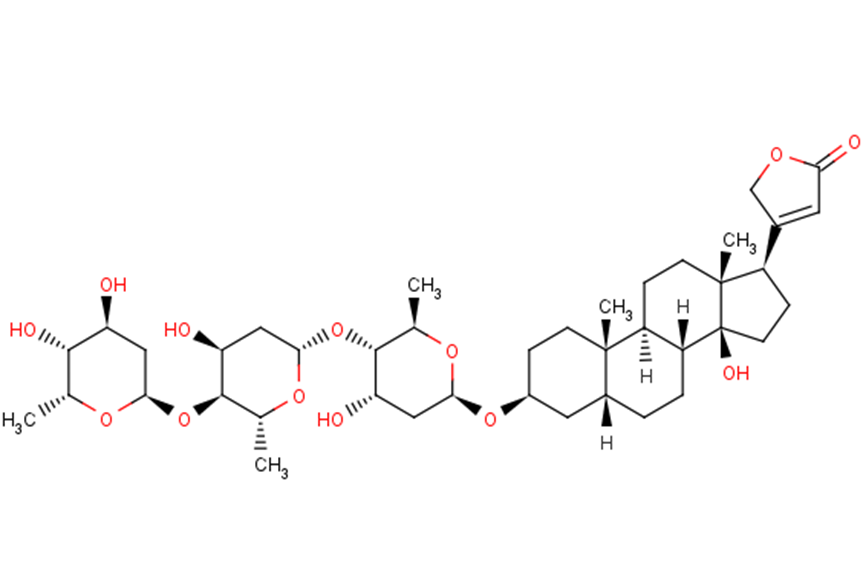
Digitoxin is a cardenolide glycoside in which the 3beta-hydroxy group of digitoxigenin carries a 2,6-dideoxy-beta-D-ribo-hexopyranosyl-(1->4)-2,6-dideoxy-beta-D-ribo-hexopyranosyl-(1->4)-2,6-dideoxy-beta-D-ribo-hexopyranosyl trisaccharide chain. It has a role as an EC 3.6.3.9 (Na(+)/K(+)-transporting ATPase) inhibitor. It is functionally related to a digitoxigenin. It is a conjugate acid of a digitoxin(1-). A cardiac glycoside sometimes used in place of digoxin. It has a longer half-life than digoxin; toxic effects, which are similar to those of digoxin, are longer lasting. (From Martindale, The Extra Pharmacopoeia, 30th ed, p665) Digitoxin has been reported in Digitalis viridiflora, Digitalis grandiflora, and other organisms with data available. Digitoxin is a lipid soluble cardiac glycoside that inhibits the plasma membrane sodium potassium ATPase, leading to increased intracellular sodium and calcium levels and decreased intracellular potassium levels. In studies increased intracellular calcium precedes cell death and decreased intracellular potassium increase caspase activation and DNA fragmentation, causing apoptosis and inhibition of cancer cell growth. (NCI) Digitoxin is only found in individuals that have used or taken this drug. It is a cardiac glycoside sometimes used in place of digoxin. It has a longer half-life than digoxin; toxic effects, which are similar to those of digoxin, are longer lasting. (From Martindale, The Extra Pharmacopoeia, 30th ed, p665)Digitoxin inhibits the Na-K-ATPase membrane pump, resulting in an increase in intracellular sodium and calcium concentrations. Increased intracellular concentrations of calcium may promote activation of contractile proteins (e.g., actin, myosin). Digitoxin also acts on the electrical activity of the heart, increasing the slope of phase 4 depolarization, shortening the action potential duration, and decreasing the maximal diastolic potential. A cardiac glycoside sometimes used in place of DIGOXIN. It has a longer half-life than digoxin; toxic effects, which are similar to those of digoxin, are longer lasting. (From Martindale, The Extra Pharmacopoeia, 30th ed, p665) See also: Acetyldigitoxin (is active moiety of); Digitalis (annotation moved to). Drug Indication For the treatment and management of congestive cardiac insufficiency, arrhythmias and heart failure. Mechanism of Action Digitoxin inhibits the Na-K-ATPase membrane pump, resulting in an increase in intracellular sodium and calcium concentrations. Increased intracellular concentrations of calcium may promote activation of contractile proteins (e.g., actin, myosin). Digitoxin also acts on the electrical activity of the heart, increasing the slope of phase 4 depolarization, shortening the action potential duration, and decreasing the maximal diastolic potential. AIMS: Recent studies suggest that proarrhythmic effects of cardiac glycosides (CGs) on cardiomyocyte Ca(2+) handling involve generation of reactive oxygen species (ROS). However, the specific pathway(s) of ROS production and the subsequent downstream molecular events that mediate CG-dependent arrhythmogenesis remain to be defined. METHODS AND RESULTS: We examined the effects of digitoxin (DGT) on Ca(2+) handling and ROS production in cardiomyocytes using a combination of pharmacological approaches and genetic mouse models. Myocytes isolated from mice deficient in NADPH oxidase type 2 (NOX2KO) and mice transgenically overexpressing mitochondrial superoxide dismutase displayed markedly increased tolerance to the proarrhythmic action of DGT as manifested by the inhibition of DGT-dependent ROS and spontaneous Ca(2+) waves (SCW). Additionally, DGT-induced mitochondrial membrane potential depolarization was abolished in NOX2KO cells. DGT-dependent ROS was suppressed by the inhibition of PI3K, PKC, and the mitochondrial KATP channel, suggesting roles for these proteins, respectively, in activation of NOX2 and in mitochondrial ROS generation. Western blot analysis revealed increased levels of oxidized CaMKII in WT but not in NOX2KO hearts treated with DGT. The DGT-induced increase in SCW frequency was abolished in myocytes isolated from mice in which the Ser 2814 CaMKII phosphorylation site on RyR2 is constitutively inactivated. CONCLUSION: These results suggest that the arrhythmogenic adverse effects of CGs on Ca(2+) handling involve PI3K- and PKC-mediated stimulation of NOX2 and subsequent NOX2-dependent ROS release from the mitochondria; mitochondria-derived ROS then activate CaMKII with consequent phosphorylation of RyR2 at Ser 2814. Pro-inflammatory processes initiated in the endothelium represent a crucial step in the pathogenesis of inflammatory cardiovascular disease, such as atherosclerosis. Recent observations pointed to potential anti-inflammatory properties of the cardiac glycoside digitoxin. Therefore, the present study investigated potential anti-inflammatory and vasoprotective properties of digitoxin as well as the underlying signaling pathways affected in endothelial cells (EC). Digitoxin, employing therapeutical concentrations used in patients (3-30 nM), potently inhibited the IL-1beta-induced expression of MCP-1 and VCAM-1 in EC and the capacity of corresponding cell culture supernatants on monocyte migration as well as monocyte adhesion to endothelial monolayers, respectively. Furthermore, digitoxin prevented the IL-1beta-induced activation of p44/42-MAPK and NF-kappaB without affecting activation of JNK and p38-MAPK. Inhibition of NF-kappaB signaling but not p44/42-MAPK mimicked the observed inhibitory effects of digitoxin on MCP-1 expression and monocyte migration. Moreover, digitoxin inhibited NF-kappaB signaling at the level of TAK-1/IKK. Additionally, digitoxin prevented TNF-alpha-induced apoptosis in EC accompanied by activation of Akt. Blockade of PI-3-kinase, activator of Akt, prevented the anti-apoptotic properties of digitoxin and impaired its inhibitory action on NF-kappaB signaling and MCP-1 expression. Finally, digitoxin activated endothelial NO-synthase, which was blocked by inhibition of PI-3-kinase, Ca(2+)/Calmodulin-dependent-proteinkinase-II and chelation of intracellular calcium. Digitoxin elicits anti-inflammatory and vasoprotective properties by blocking NF-kappaB and activating PI-3-kinase/Akt signaling as well as Ca(2+)/Calmodulin-dependent-proteinkinase-II in EC. These observations indicate a potential therapeutical application of digitoxin in the treatment of cardiovascular diseases, such as atherosclerosis. Cardiac glycosides have been used in the treatment of arrhythmias for more than 200 years. Two-pore-domain (K2P) potassium channels regulate cardiac action potential repolarization. Recently, K2P3.1 [tandem of P domains in a weak inward rectifying K+ channel (TWIK)-related acid-sensitive K+ channel (TASK)-1] has been implicated in atrial fibrillation pathophysiology and was suggested as an atrial-selective antiarrhythmic drug target. We hypothesized that blockade of cardiac K2P channels contributes to the mechanism of action of digitoxin and digoxin. All functional human K2P channels were screened for interactions with cardiac glycosides. Human K2P channel subunits were expressed in Xenopus laevis oocytes, and voltage clamp electrophysiology was used to record K+ currents. Digitoxin significantly inhibited K2P3.1 and K2P16.1 channels. By contrast, digoxin displayed isolated inhibitory effects on K2P3.1. K2P3.1 outward currents were reduced by 80% (digitoxin, 1 Hz) and 78% (digoxin, 1 Hz). Digitoxin inhibited K2P3.1 currents with an IC50 value of 7.4 uM. Outward rectification properties of the channel were not affected. Mutagenesis studies revealed that amino acid residues located at the cytoplasmic site of the K2P3.1 channel pore form parts of a molecular binding site for cardiac glycosides. In conclusion, cardiac glycosides target human K2P channels. The antiarrhythmic significance of repolarizing atrial K2P3.1 current block by digoxin and digitoxin requires validation in translational and clinical studies. Cardiac glycosides inhibit the activity of sodium-potassium-activated adenosine triphosphatase (Na+-K+-ATPase), an enzyme required for active transport of sodium across myocardial cell membranes. Inhibition of this enzyme in cardiac cells results in an increase in the contractile state of the heart and it was believed that benefits of cardiac glycosides in heart failure were mainly associated with inotropic action. However, it has been suggested that benefits of cardiac glycosides may be in part related to enzyme inhibition in noncardiac tissues. Inhibition of Na+-K+-ATPase in vagal afferents acts to sensitize cardiac baroreceptors which may in turn decrease sympathetic outflow from the CNS. In addition, by inhibiting Na+-K+-ATPase in the kidney, cardiac glycosides decrease the renal tubular reabsorption of sodium; the resulting increase in the delivery of sodium to the distal tubules leads to the suppression of renin secretion from the kidneys. These observations led to the hypothesis that cardiac glycosides act in heart failure principally by attenuating the activation of the neurohormonal system, rather than by a positive inotropic action. Toxic doses of cardiac glycosides cause efflux of potassium from the myocardium and concurrent influx of sodium. Toxicity results in part from loss of intracellular potassium associated with inhibition of Na+-K+-ATPase. With therapeutic doses, augmentation of calcium influx to the contractile proteins with resultant enhancement of excitation-contraction coupling is involved in the positive inotropic action of cardiac glycosides; the role of Na+-K+-ATPase in this effect is controversial. /Cardiac glycosides/ Low concentrations of cardiac glycosides including ouabain, digoxin, and digitoxin block cancer cell growth without affecting Na+,K+-ATPase activity, but the mechanism underlying this anti-cancer effect is not fully understood. Volume-regulated anion channel (VRAC) plays an important role in cell death signaling pathway in addition to its fundamental role in the cell volume maintenance. Here, we report cardiac glycosides-induced signaling pathway mediated by the crosstalk between Na+,K+-ATPase and VRAC in human cancer cells. Submicromolar concentrations of ouabain enhanced VRAC currents concomitantly with a deceleration of cancer cell proliferation. The effects of ouabain were abrogated by a specific inhibitor of VRAC (DCPIB) and knockdown of an essential component of VRAC (LRRC8A), and they were also attenuated by the disruption of membrane microdomains or the inhibition of NADPH oxidase. Digoxin and digitoxin also showed anti-proliferative effects in cancer cells at their therapeutic concentration ranges, and these effects were blocked by DCPIB. In membrane microdomains of cancer cells, LRRC8A was found to be co-immunoprecipitated with Na+,K+-ATPase a1-isoform. These ouabain-induced effects were not observed in non-cancer cells. Therefore, cardiac glycosides were considered to interact with Na+,K+-ATPase to stimulate the production of reactive oxygen species, and they also apparently activated VRAC within membrane microdomains, thus producing anti-proliferative effects. - Mechanism of action: Digitoxin exerts its effects through multiple mechanisms: forming transmembrane calcium channels (regulating intracellular calcium), inhibiting HSV DNA polymerase (blocking viral replication), and inducing cancer cell cycle arrest/apoptosis via cyclin B1 downregulation [1,2,3]. - Therapeutic potential: Investigated for heart failure (original indication), antiviral therapy (HSV, influenza), and cancer (cervical, lung) due to its selective cytotoxicity and cytokine-modulating properties [1,2,3,4,5]. |
| 分子式 |
C41H64O13
|
|---|---|
| 分子量 |
764.95
|
| 精确质量 |
764.434
|
| 元素分析 |
C, 64.38; H, 8.43; O, 27.19
|
| CAS号 |
71-63-6
|
| 相关CAS号 |
Gitoxin;4562-36-1
|
| PubChem CID |
441207
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
902.3±65.0 °C at 760 mmHg
|
| 熔点 |
240ºC (dec.)(lit.)
|
| 闪点 |
269.5±27.8 °C
|
| 蒸汽压 |
0.0±0.6 mmHg at 25°C
|
| 折射率 |
1.594
|
| LogP |
2.44
|
| tPSA |
182.83
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
13
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
54
|
| 分子复杂度/Complexity |
1410
|
| 定义原子立体中心数目 |
20
|
| SMILES |
C[C@@H]1[C@H]([C@H](C[C@@H](O1)O[C@@H]2[C@H](O[C@H](C[C@@H]2O)O[C@@H]3[C@H](O[C@H](C[C@@H]3O)O[C@H]4CC[C@]5([C@@H](C4)CC[C@@H]6[C@@H]5CC[C@]7([C@@]6(CC[C@@H]7C8=CC(=O)OC8)O)C)C)C)C)O)O
|
| InChi Key |
WDJUZGPOPHTGOT-XUDUSOBPSA-N
|
| InChi Code |
InChI=1S/C41H64O13/c1-20-36(46)29(42)16-34(49-20)53-38-22(3)51-35(18-31(38)44)54-37-21(2)50-33(17-30(37)43)52-25-8-11-39(4)24(15-25)6-7-28-27(39)9-12-40(5)26(10-13-41(28,40)47)23-14-32(45)48-19-23/h14,20-22,24-31,33-38,42-44,46-47H,6-13,15-19H2,1-5H3/t20-,21-,22-,24-,25+,26-,27+,28-,29+,30+,31+,33+,34+,35+,36-,37-,38-,39+,40-,41+/m1/s1
|
| 化学名 |
3-[(3S,5R,8R,9S,10S,13R,14S,17R)-3-[(2R,4S,5S,6R)-5-[(2S,4S,5S,6R)-5-[(2S,4S,5S,6R)-4,5-dihydroxy-6-methyloxan-2-yl]oxy-4-hydroxy-6-methyloxan-2-yl]oxy-4-hydroxy-6-methyloxan-2-yl]oxy-14-hydroxy-10,13-dimethyl-1,2,3,4,5,6,7,8,9,11,12,15,16,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]-2H-furan-5-one
|
| 别名 |
NSC 7529; digitoxin; 71-63-6; Digitoxoside; Crystodigin; Digitoxinum; Unidigin; Digitophyllin; Carditoxin; NSC-7529; NSC7529; Carditalin;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~130.73 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (3.27 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (3.27 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (3.27 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3073 mL | 6.5364 mL | 13.0727 mL | |
| 5 mM | 0.2615 mL | 1.3073 mL | 2.6145 mL | |
| 10 mM | 0.1307 mL | 0.6536 mL | 1.3073 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。