| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
| 靶点 |
γ-Glutamyl transpeptidase (γ-GT) [2]
- Renal drug transporters (OAT1, OAT3, OCT2, MATE1) [3] - Microtubule proteins (tubulin) [1] |
|---|---|
| 体外研究 (In Vitro) |
Dimesna 以剂量依赖性方式调节紫杉醇诱导的 MTP 高聚合,而 Mesna(Dimesna 的体内代谢物)可防止时间依赖性顺铂诱导的 MTP 失活。 Dimesna介导的顺铂诱导的肾毒性的预防或减轻可能涉及某些Dimesna衍生的esna-二硫化物杂合物对氨肽酶N(APN)的抑制,并且似乎与甘氨酸部分和/或阴离子基团的存在相关。提出了 Dimesna 介导的顺铂诱导的肾毒性的肾保护的两种一般机制,涉及 γ-谷氨酰转肽酶 (GGT)、APN 和半胱氨酸缀合-β-裂合酶 (CCBL) 肾毒性途径,它们以协调和/或协同的方式起作用,从而预防或减轻顺铂引起的肾毒性。 Mesna 及其二聚体 Dimesna 联合用药分别可减轻异环磷酰胺和顺铂引起的毒性。迪美司钠在肾脏中选择性还原为美司钠,产生保护作用。摄取和流出转运蛋白的体外筛选显示肾脏有机阴离子转运蛋白 OAT1、OAT3 和 OAT4 负责 Dimesna 的肾脏特异性摄取。 OAT1、OAT3 和 OAT4 对 Dimesna 的摄取被确定为饱和,KM 分别为 636 μM、390 μM 和 590 μM。
Dimesna(BNP-7787)在体外调节紫杉醇和顺铂诱导的微管蛋白聚合异常。它可逆转紫杉醇诱导的微管过度稳定化及顺铂诱导的微管解聚,恢复正常微管动力学,且在无化疗药物存在时不会直接改变微管蛋白聚合[1] - Dimesna(BNP-7787)通过抑制肾近端小管细胞中的γ-GT活性发挥肾保护作用。它减少顺铂诱导的活性氧(ROS)产生和细胞凋亡,该效应与降低γ-GT介导的顺铂毒性激活相关[2] - Dimesna(BNP-7787)是肾有机阴离子转运体(OAT1、OAT3)和有机阳离子转运体(OCT2、MATE1)的底物。在过表达OAT1/OAT3的细胞中呈浓度依赖性摄取,其转运可被特异性OAT/OCT/MATE抑制剂阻断。未观察到明显的血浆蛋白结合(蛋白结合率<10%)[3] |
| 体内研究 (In Vivo) |
给予 Dimesna 可以以剂量相关的方式显着减少环磷酰胺 (CP) 诱导的大鼠膀胱肿瘤。
在顺铂诱导的肾毒性大鼠模型中,静脉注射Dimesna(BNP-7787)(150 mg/kg,顺铂给药前30分钟)降低血清肌酐和血尿素氮(BUN)水平,减轻肾小管坏死,减少肾组织中ROS生成和凋亡细胞数量。它下调肾组织中γ-GT的表达,抑制顺铂诱导的γ-GT激活[2] - 在环磷酰胺处理的大鼠中,Dimesna(BNP-7787)(100 mg/kg,腹腔注射,环磷酰胺给药前30分钟和给药后4小时各1次)显著降低膀胱肿瘤发生率。它预防环磷酰胺诱导的膀胱黏膜损伤和氧化应激,且不影响环磷酰胺的抗肿瘤疗效[4] - 大鼠静脉给药后,Dimesna(BNP-7787)快速分布至肾脏和膀胱,主要以原型形式经尿液排泄,肾清除率由OAT1/OAT3和OCT2/MATE1转运体介导[3] |
| 酶活实验 |
γ-GT活性实验:将纯化的γ-GT酶与γ-谷氨酰-p-硝基苯胺(底物)及不同浓度的Dimesna(BNP-7787)在37°C下孵育60分钟。分光光度法在405 nm处检测p-硝基苯胺的释放量,计算γ-GT抑制率[2]
- 微管聚合实验:将纯化的微管蛋白与紫杉醇或顺铂在有或无Dimesna(BNP-7787)的条件下孵育,通过60分钟内340 nm处浊度变化监测微管聚合动力学,量化聚合/解聚程度[1] |
| 细胞实验 |
肾近端小管细胞实验:细胞用0-500 μM Dimesna(BNP-7787)预处理2小时后,暴露于20 μM顺铂中24小时。MTT法检测细胞活力;荧光探针染色检测ROS生成;Annexin V-FITC/PI双染色检测细胞凋亡。Western blot检测γ-GT及凋亡相关蛋白(Bcl-2、Bax、caspase-3)的表达[2]
- 肾转运体细胞实验:将过表达OAT1、OAT3、OCT2或MATE1的HEK293细胞与[³H]标记的Dimesna(BNP-7787)(0-100 μM)在37°C下孵育30分钟。闪烁计数法检测细胞相关放射性,评估转运体介导的摄取。通过与特异性转运体抑制剂共孵育进行抑制实验[3] - 微管细胞实验:HeLa细胞单独用紫杉醇(10 nM)或顺铂(50 μM)处理,或与100-500 μM Dimesna(BNP-7787)联合处理24小时。微管蛋白特异性抗体免疫荧光染色可视化微管结构,荧光显微镜下观察形态学变化[1] |
| 动物实验 |
Dissolved in drinking water; 12 or 35 mg/kg ; Oral administration
CP treated Sprague-Dawley rats Cisplatin nephrotoxicity model: Male rats were randomly divided into control, cisplatin, and Dimesna (BNP-7787) + cisplatin groups. Dimesna (BNP-7787) was dissolved in normal saline and administered intravenously at 150 mg/kg 30 minutes before cisplatin (7 mg/kg, intravenous injection). Rats were sacrificed 72 hours after cisplatin administration; serum and renal tissues were collected for biochemical and histological analysis [2] - Bladder tumor prevention model: Female rats were treated with cyclophosphamide (50 mg/kg, intraperitoneal injection) once weekly for 10 weeks. Dimesna (BNP-7787) was dissolved in normal saline and administered intraperitoneally at 100 mg/kg 30 minutes before and 4 hours after each cyclophosphamide injection. Rats were sacrificed 24 weeks after the first cyclophosphamide dose; bladder tissues were collected for tumor incidence analysis and histological examination [4] - Renal transporter disposition model: Rats were administered [³H]-labeled Dimesna (BNP-7787) (5 mg/kg, intravenous injection). Blood, urine, and kidney tissues were collected at different time points (0-24 hours). Radioactivity in samples was measured by scintillation counting to determine pharmacokinetic parameters and renal excretion efficiency [3] |
| 药代性质 (ADME/PK) |
Dimesna (BNP-7787) showed rapid absorption and distribution after intravenous administration in rats, with a plasma half-life (t1/2) of ~1.2 hours [3]
- It was primarily excreted unchanged in urine, with ~85% of the administered dose recovered in urine within 24 hours [3] - Renal clearance was mediated by OAT1/OAT3 (basolateral membrane) and OCT2/MATE1 (apical membrane) transporters, facilitating its accumulation in renal tubules and bladder [3] - Plasma protein binding was < 10%, with no significant binding to albumin or other plasma proteins [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
In vitro, Dimesna (BNP-7787) showed no cytotoxicity to normal renal cells, HeLa cells, or bladder epithelial cells at concentrations up to 1000 μM [1][2][4]
- In vivo, administration of Dimesna (BNP-7787) (doses up to 500 mg/kg in animal models) did not cause significant changes in body weight, organ index, or serum liver/kidney function indicators, indicating low systemic toxicity [2][3][4] - No drug-drug interactions were observed when co-administered with cisplatin, paclitaxel, or cyclophosphamide [1][2][4] |
| 参考文献 |
|
| 其他信息 |
Dimesna is the disodium salt form of dithio-ethane sulfonate, a dimer of mesna and a disulfide bond disrupting agent (DDA), with uroprotective, nephroprotective, chemoprotective, chemosensitizing and chemo-enhancing activities. Upon administration, dimesna is able to modify cysteine on various proteins, such as the kinases EGFR, MET and ROS1, thereby disrupting extracellular disulfide bonds and modulating the activity of these proteins. This inhibits their signaling pathways and downregulates proliferative signaling in cancer cells in which these kinases are overexpressed. This may also enhance the activity of other kinase inhibitors targeting the same proteins. In the kidneys, dimesna undergoes reduction to the free thiol compound, mesna, which reacts chemically with the urotoxic ifosfamide metabolites acrolein and 4-hydroxy-ifosfamide, resulting in their detoxification. This agent also inhibits cyclophosphamide-induced hemorrhagic cystitis. In addition, dimesna reduces toxicities associated with taxanes and platinum-based chemotherapeutic agents.
Dimesna (BNP-7787) is a synthetic sulfhydryl-containing compound and the disulfide form of mesna. It acts as a chemoprotective agent, mitigating toxic effects of chemotherapeutics (cisplatin, cyclophosphamide, paclitaxel) without compromising their antitumor efficacy [1][2][4] - Its nephroprotective mechanism involves inhibition of γ-GT-mediated activation of cisplatin and scavenging of cisplatin-induced ROS [2] - Uroprotective effects are attributed to direct binding to cyclophosphamide-derived toxic metabolites in the bladder, preventing mucosal damage and tumorigenesis [4] - Modulation of microtubule dynamics helps reduce paclitaxel/cisplatin-induced neurotoxicity and myelosuppression [1] |
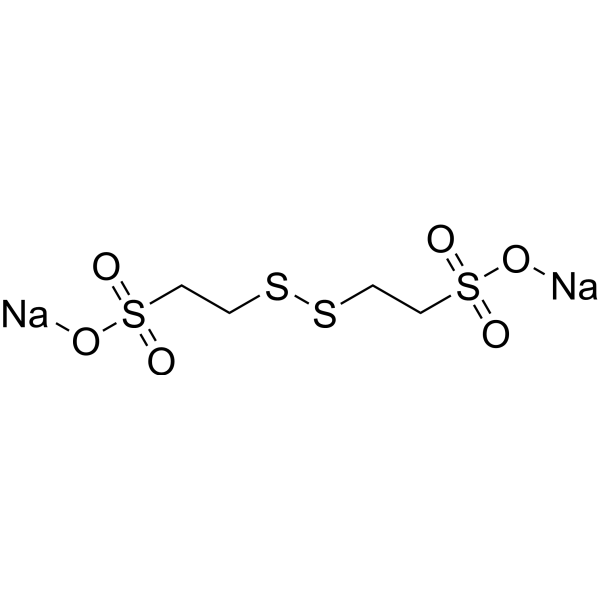
| 分子式 |
C4H8NA2O6S4
|
|
|---|---|---|
| 分子量 |
326.34
|
|
| 精确质量 |
325.899
|
|
| CAS号 |
16208-51-8
|
|
| 相关CAS号 |
|
|
| PubChem CID |
65625
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| LogP |
1.619
|
|
| tPSA |
181.76
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
8
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
16
|
|
| 分子复杂度/Complexity |
268
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
S(CCSSCCS(=O)(=O)[O-])(=O)(=O)[O-].[Na+].[Na+]
|
|
| InChi Key |
KQYGMURBTJPBPQ-UHFFFAOYSA-L
|
|
| InChi Code |
InChI=1S/C4H10O6S4.2Na/c5-13(6,7)3-1-11-12-2-4-14(8,9)10;;/h1-4H2,(H,5,6,7)(H,8,9,10);;/q;2*+1/p-2
|
|
| 化学名 |
disodium;2-(2-sulfonatoethyldisulfanyl)ethanesulfonate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|---|
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0643 mL | 15.3214 mL | 30.6429 mL | |
| 5 mM | 0.6129 mL | 3.0643 mL | 6.1286 mL | |
| 10 mM | 0.3064 mL | 1.5321 mL | 3.0643 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。