| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| Other Sizes |
|
| 靶点 |
H1 Receptor
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Diphenhydramine is quickly absorbed after oral administration with maximum activity occurring in approximately one hour. The oral bioavailability of diphenhydramine has been documented in the range of 40% to 60%, and peak plasma concentration occurs about 2 to 3 hours after administration. The metabolites of diphenhydramine are conjugated with glycine and glutamine and excreted in urine. Only about 1% of a single dose is excreted unchanged in urine. The medication is ultimately eliminated by the kidneys slowly, mainly as inactive metabolites. Diphenhydramine is widely distributed throughout the body, including the CNS. Following a 50 mg oral dose of diphenhydramine, the volume of distribution is in the range of 3.3 - 6.8 l/kg. Values for plasma clearance of a 50 mg oral dose of diphenhydramine has been documented as lying in the range of 600-1300 ml/min. Distribution of diphenhydramine into human body tissues and fluids has not been fully characterized. Following IV administration in rats, highest concentrations of the drug are attained in the lungs, spleen, and brain, with lower concentrations in the heart, muscle, and liver. Following IV administration in healthy adults, diphenhydramine reportedly has an apparent volume of distribution of 188-336 L. Volume of distribution of the drug reportedly is larger in Asian (about 480 L) than white adults. The drug crosses the placenta and has been detected in milk, although the extent of distribution into milk has not been quantitated. Following oral administration of a single 100-mg dose in healthy adults, about 50-75% of the dose is excreted in urine within 4 days, almost completely as metabolites and with most urinary excretion occurring within the first 24-48 hours; only about 1% of a single oral dose is excreted unchanged in urine. Diphenhydramine, given orally, reaches a maximal concentration in the blood in approximately 2 hours, remains there for another 2 hours, then falls exponentially with a plasma elimination half life of approximately 4-8 hours. The drug is distributed widely throughout the body, including the CNS. Little, if any is excreted unchanged in the urine; most appears there as metabolites. For more Absorption, Distribution and Excretion (Complete) data for DIPHENHYDRAMINE (7 total), please visit the HSDB record page. Metabolism / Metabolites Diphenhydramine undergoes rapid and extensive first-pass metabolism. In particular, two successive N-demethylations occur wherein diphenhydramine is demethylated to N-desmethyldiphenhydramine (the N-desmethyl metabolite) and then this metabolite is itself demethylated to N,N-didesmethyldiphenhydramine (the N,N-didesmethyl metabolite). Subsequently, acetyl metabolites like N-acetyl-N-desmethyldiphenhydramine are generated via the amine moiety of the N,N-didesmethyl metabolite. Additionally, the N,N-didesmethyl metabolite also undergoes some oxidation to generate the diphenylmethoxyacetic acid metabolite as well. The remaining percentage of a dose of administered diphenhydramine is excreted unchanged. The metabolites are further conjugated with glycine and glutamine and excreted in urine. Moreover, studies have determined that a variety of cytochrome P450 isoenzymes are involved in the N-demethylation that characterizes the primary metabolic pathway of diphenhydramine, including CYP2D6, CYP1A2, CYP2C9, and CYP2C19. In particular, CYP2D6 demonstrates higher affinity catalysis with the diphenhydramine substrate than the other isoenzymes identified. Consequently, inducers or inhibitors of these such CYP enzymes may potentially affect the serum concentration and incidence and/or severity of adverse effects associated with exposure to diphenhydramine. Diphenhydramine is rapidly and apparently almost completely metabolized. Following oral administration, the drug apparently undergoes substantial first-pass metabolism in the liver. Diphenhydramine appears to be metabolized principally to diphenylmethoxyacetic acid, which may further undergo conjugation. The drug also undergoes dealkylation to form the N-demethyl and N, N-didemethyl derivatives. Diphenhydramine and its metabolites are excreted principally in urine. Diphenhydramine is widely used as an over-the-counter antihistamine. However, the specific human cytochrome P450 (P450) isozymes that mediate the metabolism of diphenhydramine in the range of clinically relevant concentrations (0.14-0.77 microM) remain unclear. Therefore, P450 isozymes involved in N-demethylation, a main metabolic pathway of diphenhydramine, were identified by a liquid chromatography-mass spectrometry method developed in our laboratory. Among 14 recombinant P450 isozymes, CYP2D6 showed the highest activity of diphenhydramine N-demethylation (0.69 pmol/min/pmol P450) at 0.5 uM. CYP2D6 catalyzed diphenhydramine N-demethylation as a high-affinity P450 isozyme, the K(m) value of which was 1.12 +/- 0.21 uM. In addition, CYP1A2, CYP2C9, and CYP2C19 were identified as low-affinity components. In human liver microsomes, involvement of CYP2D6, CYP1A2, CYP2C9, and CYP2C19 in diphenhydramine N-demethylation was confirmed by using P450 isozyme-specific inhibitors. In addition, contributions of these P450 isozymes estimated by the relative activity factor were in good agreement with the results of inhibition studies. Although an inhibitory effect of diphenhydramine on the metabolic activity of CYP2D6 has been reported previously, the results of the present study suggest that it is not only a potent inhibitor but also a high-affinity substrate of CYP2D6. Therefore, it is worth mentioning that the sedative effect of diphenhydramine might be caused by coadministration of CYP2D6 substrate(s)/inhibitor(s). In addition, large differences in the metabolic activities of CYP2D6 and those of CYP1A2, CYP2C9, and CYP2C19 could cause the individual differences in anti-allergic efficacy and the sedative effect of diphenhydramine. Two strains of the filamentous fungus Cunninghamella elegans (ATCC 9245 and ATCC 36112) were grown in Sabouraud dextrose broth and screened for the ability to metabolize the ethanolamine-type antihistamine diphenhydramine. Based on the amount of parent drug recovered after 7 days incubation, both C. elegans strains metabolized approximately 74% of the diphenhydramine, 58% of this being identified as organic extractable metabolites. The organic extractable metabolites were isolated by reversed-phase high-performance liquid chromatography and identified by analyzing their mass and nuclear magnetic resonance spectra. Desorption chemical ionization mass spectrometry (DCIMS) with deuterated ammonia was used to differentiate possible isobaric diphenhydramine metabolites and to probe the mechanisms of ion formation under ammonia DCIMS conditions. C. elegans transformed diphenhydramine by demethylation, oxidation, and N-acetylation. The major metabolites observed were diphenhydramine-N-oxide (3%), N-desmethyldiphenhydramine (30%), N-acetyldidesmethyldiphenhydramine (13%), and N-acetyl-N-desmethyldiphenhydramine (12%). These compounds are known mammalian metabolites of diphenhydramine ... . Diphenhydramine has known human metabolites that include Diphenhydramine N-glucuronide and N-Desmethyldiphenhydramine. Hepatic and renal Route of Elimination: Little, if any, is excreted unchanged in the urine; most appears as the degradation products of metabolic transformation in the liver, which are almost completely excreted within 24 hours. Half Life: 1-4 hours Biological Half-Life The elimination half-life ranges from 2.4-9.3 hours in healthy adults. The terminal elimination half-life is prolonged in liver cirrhosis. The pharmacokinetics and pharmacodynamics of the H1-receptor antagonist diphenhydramine were studied in 21 fasting subjects divided into three age groups: elderly, (mean age 69.4 +/- 4.3 years), young adults, (mean age 31.5 +/- 10.4 years), and children, (mean age 8.9 +/- 1.7 years). All subjects ingested a single dose of diphenhydramine syrup 1.25 mg/kg. ... The mean serum elimination half-life values for diphenhydramine differed significantly in elderly adults, young adults, and children, with values of 13.5 +/- 4.2 hours, 9.2 +/- 2.5 hours, and 5.4 +/- 1.8 hours being found respectively in each age group. ... The terminal elimination half-life of diphenhydramine has not been fully elucidated, but appears to range from 2.4-9.3 hours in healthy adults. The terminal elimination half-life reportedly is prolonged in adults with liver cirrhosis. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Diphenhydramine competes with free histamine for binding at HA-receptor sites. This antagonizes the effects of histamine on HA-receptors, leading to a reduction of the negative symptoms brought on by histamine HA-receptor binding. Toxicity Data LD50: 500 mg/kg (Oral, Rat) (A308) Interactions Concurrent use /of ototoxic medications/ with antihistamines may mask the symptoms of ototoxicity such as tinnitus, dizziness, or vertigo. /Antihistamines/ Concurrent use of monoamine oxidase (MAO) inhibitors with antihistamines may prolong and intensify the anticholinergic and CNS depressant effects of antihistamines; concurrent use is not recommended. /Antihistamines/ Concurrent use /with alcohol or other CNS depression-producing medications/ may potentiate the CNS depressant effects of either these medications or antihistamines; also, concurrent use of maprotiline or tricyclic antidepressants may potentiate the anticholinergic effects of either antihistamines or these medications. /Antihistamines/ Anticholinergic effects may be potentiated when /anticholinergics or other medications with anticholinergic activity/ are used concurrently with antihistamines; patients should be advised to report occurrence of gastrointestinal problems promptly since paralytic ileus may occur with concurrent therapy. /Antihistamines/ For more Interactions (Complete) data for DIPHENHYDRAMINE (8 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Anesthetics, Local; Anti-Allergic Agents; Antiemetics; Histamine H1 Antagonists; Hypnotics and Sedatives Antihistamines are most beneficial in the management of nasal allergies. Seasonal allergic rhinitis (e.g., hay fever) and perennial (nonseasonal) allergic rhinitis are benefited more than perennial nonallergic (vasomotor) rhinitis. Orally administered antihistamines generally provide symptomatic relief of rhinorrhea, sneezing, oronasopharyngeal irritation or itching, lacrimation, and red, irritated, or itching eyes associated with the early response to histamine. /Antihistamines; Included in US product labeling/ Antihistamines are often effective in the treatment of allergic dermatoses and other dermatoses associated with histamine release, but effectiveness varies with the causative agent and symptoms may return when the drug is stopped. /Antihistamines; Included in US product labeling/ Antihistamines may provide some benefit in certain asthmatic patients, but the drugs usually are not effective in treating bronchial asthma per se and should not be used in the treatment of severe acute asthma attacks. In addition, antihistamines are not included in the usual recommended regimens for the management of asthma, including long-term control of the disease. /Antihistamines; Included in US product labeling/ For more Therapeutic Uses (Complete) data for DIPHENHYDRAMINE (12 total), please visit the HSDB record page. Drug Warnings Numerous side effects ... /incl/ drowsiness, confusion, restlessness, nausea, vomiting, diarrhea, blurring of vision, diplopia, difficulty in urination, constipation, nasal stuffiness, vertigo, palpitation, headache, and insomnia. Other side effects observed were urticaria, drug rash, photosensitivity, hemolytic anemia, hypotension, epigastric distress, anaphylactic shock, tightness of the chest and wheezing, thickening of bronchial secretions, dryness of the mouth, nose and throat and tingling, and heaviness and weakness of the hands. Like other antihistamines, diphenhydramine should be used with caution in infants and young children and should not be used in premature or full-term neonates Children younger than 6 years of age should receive diphenhydramine only under the direction of a physician. Safety and efficacy of diphenhydramine as a nighttime sleep aid in children younger than 12 years of age have not been established. In addition, children may be more prone than adults to paradoxically experience CNS stimulation rather than sedation when antihistamines are used as nighttime sleep aids. Because diphenhydramine may cause marked drowsiness that may be potentiated by other CNS depressants (e.g., sedatives, tranquilizers), the antihistamine should be used in children receiving one of these drugs only under the direction of a physician. Prolonged use of antihistamines ... may decrease or inhibit salivary flow, thus contributing to the development of caries, periodontal disease, oral candidiasis, and discomfort. /Antihistamines/ Local necrosis has occurred with subcutaneous or intradermal administration of parenteral diphenhydramine. For more Drug Warnings (Complete) data for DIPHENHYDRAMINE (18 total), please visit the HSDB record page. Pharmacodynamics Diphenhydramine has anti-histaminic (H1-receptor), anti-emetic, anti-vertigo and sedative and hypnotic properties. The anti-histamine action occurs by blocking the spasmogenic and congestive effects of histamine by competing with histamine for H1 receptor sites on effector cells, preventing but not reversing responses mediated by histamine alone. Such receptor sites may be found in the gut, uterus, large blood vessels, bronchial muscles, and elsewhere. Anti-emetic action is by inhibition at the medullary chemoreceptor trigger zone. Anti-vertigo action is by a central antimuscarinic effect on the vestibular apparatus and the integrative vomiting center and medullary chemoreceptor trigger zone of the midbrain. |
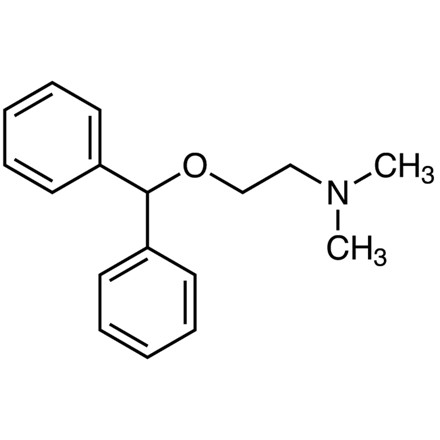
| 分子式 |
C17H21NO
|
|---|---|
| 分子量 |
255.3547
|
| 精确质量 |
255.162
|
| 元素分析 |
C, 79.96; H, 8.29; N, 5.49; O, 6.27
|
| CAS号 |
58-73-1
|
| 相关CAS号 |
Diphenhydramine hydrochloride; 147-24-0; 88637-37-0 (citrate); 7491-10-3 (salicylate)
|
| PubChem CID |
3100
|
| 外观&性状 |
Colorless to light yellow liquid
|
| 密度 |
1.0±0.1 g/cm3
|
| 沸点 |
343.7±27.0 °C at 760 mmHg
|
| 熔点 |
167-172°C
|
| 闪点 |
101.5±26.0 °C
|
| 蒸汽压 |
0.0±0.8 mmHg at 25°C
|
| 折射率 |
1.551
|
| LogP |
3.66
|
| tPSA |
12.47
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
19
|
| 分子复杂度/Complexity |
211
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O(C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])[H])C([H])(C1C([H])=C([H])C([H])=C([H])C=1[H])C1C([H])=C([H])C([H])=C([H])C=1[H]
|
| InChi Key |
ZZVUWRFHKOJYTH-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C17H21NO/c1-18(2)13-14-19-17(15-9-5-3-6-10-15)16-11-7-4-8-12-16/h3-12,17H,13-14H2,1-2H3
|
| 化学名 |
2-benzhydryloxy-N,N-dimethylethanamine
|
| 别名 |
Diphenhydramine; Debendrin; Difenhydramine; Dabylen; PM255; PM-255; PM 255
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~100 mg/mL (~391.6 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.79 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.79 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.9162 mL | 19.5810 mL | 39.1619 mL | |
| 5 mM | 0.7832 mL | 3.9162 mL | 7.8324 mL | |
| 10 mM | 0.3916 mL | 1.9581 mL | 3.9162 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04175834 | Active Recruiting |
Drug: antihistamine | Multiple Sclerosis Infusion Reaction |
Providence Health & Services | February 5, 2020 | Phase 3 |
| NCT02037126 | Active Recruiting |
Drug: Diphenhydramine Drug: Psilocybin |
Cocaine-Related Disorders | University of Alabama at Birmingham |
May 2015 | Phase 2 |
| NCT04741139 | Active Recruiting |
Drug: Acetaminophen and Diphenhydramine Only Product |
Immune Thrombocytopenia | Baylor College of Medicine | September 2, 2021 | Phase 1 |
| NCT04109885 | Active Recruiting |
Drug: Paracervical injection Drug: prochlorperazine and diphenhydramine. (Standard Treatment) |
Pain Management Emergency Department |
Christian Fromm, MD | September 15, 2020 | Phase 2 |
| NCT04805073 | Recruiting | Drug: Promethazine Drug: Placebo |
Pruritus Pregnancy Related |
University of Florida | August 9, 2021 | Phase 4 |