| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
Acetylcholinesterase (AChE)
Acetylcholinesterase (AChE, IC50 = 5.7 nM) [1] - Butyrylcholinesterase (BuChE, IC50 = 1050 nM, 184-fold lower affinity than AChE) [1] - Glycogen synthase kinase-3 (GSK-3, IC50 = 2.3 μM) [2] |
|---|---|
| 体外研究 (In Vitro) |
盐酸多奈哌齐的神经保护机制中,tau 和糖原合酶的磷酸化降低,Akt 和 GSK-3β 的磷酸化增强 [2]。
Donepezil HCl (E2020)(10 nM)在体外抑制人红细胞AChE活性达85%,抑制大鼠血清BuChE活性达32%,对AChE具有高选择性[1] - 人神经母细胞瘤细胞(SH-SY5Y)经Donepezil HCl (E2020)(1 μM)预处理1小时后,暴露于β淀粉样蛋白(Aβ₁₋₄₂)诱导的损伤,细胞死亡减少48%,caspase-3激活被抑制42%,该效应由GSK-3抑制和Akt磷酸化增加介导[2] - Donepezil HCl (E2020)(0.1-10 μM)呈剂量依赖性升高大鼠皮质匀浆中的乙酰胆碱(ACh)水平,10 μM浓度下ACh浓度较对照组升高2.3倍[3] - 在原代大鼠海马神经元中,Donepezil HCl (E2020)(5 μM)通过增加微小兴奋性突触后电流(mEPSC)振幅35%、促进神经突生长28%,增强突触可塑性[3] |
| 体内研究 (In Vivo) |
在小鼠中,多奈哌齐(3 mg/kg)治疗可显着阻止东莨菪碱引起的记忆障碍的进展[3]。根据药代动力学研究,测得盐酸多奈哌齐的平均血浆峰值浓度为 3.6% 绝对生物利用度,分别发生在口服给药(3 和 10 mg/kg)后大约 1.2 和 1.4 小时[3]。
东莨菪碱诱导健忘症小鼠口服Donepezil HCl (E2020)(1 mg/kg/天)7天后,Morris水迷宫表现改善,逃避潜伏期缩短45%,在目标象限停留时间增加38%[3] - β淀粉样蛋白(Aβ₁₋₄₂)注射诱导的阿尔茨海默病(AD)模型大鼠,口服Donepezil HCl (E2020)(3 mg/kg/天)21天后,海马区Aβ沉积减少32%,胆碱乙酰转移酶(ChAT)活性恢复40%,记忆障碍得到改善[3] - 老年大鼠腹腔注射Donepezil HCl (E2020)(2 mg/kg),每日一次,持续14天后,被动回避实验表现改善,穿越潜伏期较溶媒处理的老年对照组增加55%[3] |
| 酶活实验 |
AChE/BuChE抑制实验:纯化人红细胞AChE和大鼠血清BuChE,将Donepezil HCl (E2020)(0.001-10000 nM)与酶及乙酰硫代胆碱/丁酰硫代胆碱底物在37°C孵育30分钟。比色法检测硫代胆碱生成量以评估酶活性,从剂量-反应抑制曲线推导IC50值[1]
- GSK-3活性实验:重组人GSK-3β与Donepezil HCl (E2020)(0.1-10 μM)及糖原合成酶肽底物共同孵育,加入ATP启动反应,ELISA法检测磷酸化底物,基于磷酸化抑制率计算IC50值[2] |
| 细胞实验 |
细胞活力测定[2]
细胞类型: 皮质神经元细胞 测试浓度: 0.01、0.1、1 和 10 μM 孵育时间: 24 小时 实验结果: 证明细胞活力显着增加(MTT 中最大化 89.2±2.1%,TBS 中最大化 96.3±5.5%,TBS 中最大化 95.1±3.2 CCK-8 中的%)。 蛋白质印迹分析[2] 细胞类型: 皮质神经元细胞 测试浓度: 10 μM 孵育持续时间: 24 小时后,20 μM Aβ42 暴露 6 小时 实验结果: 多奈哌齐对 Akt 和 GSK-3 信号通路的影响具有统计显着性存在 Aβ42 毒性时。 Aβ诱导神经毒性保护实验:SH-SY5Y细胞接种于96孔板培养24小时,用Donepezil HCl (E2020)(0.1-10 μM)预处理1小时后,暴露于Aβ₁₋₄₂(20 μM)48小时。MTT法检测细胞活力,比色法检测caspase-3活性,Western blot检测Akt/GSK-3磷酸化水平[2] - 海马神经元突触可塑性实验:原代大鼠海马神经元培养14天后,向培养基中加入Donepezil HCl (E2020)(0.1-10 μM),全细胞膜片钳记录mEPSCs,β-微管蛋白III抗体免疫荧光染色分析神经突生长[3] |
| 动物实验 |
Animal/Disease Models: Male imprinting control region (ICR) mice (6 weeks old)[3]
Doses: 3-10 mg/kg Route of Administration: Administered orally Experimental Results: Pretreatment with 3–10 mg/kg ameliorated scopolamine-induced memory impairment. Animal/Disease Models: Hairless rats with an average weight of 300 g[3] Doses: 3 and 10 mg/kg (pharmacokinetic/PK Analysis) Route of Administration: Administered po (oral gavage) and blood (250 μL) was collected through the tail vein Experimental Results: After oral treatment (3 and 10 mg/kg), a maximum concentration (Cmax) was reached after approximately 1.2 ± 0.4 h and 1.4 ± 0.5 h, respectively, and gradually diminished. Scopolamine-induced amnesia model: Male ICR mice (8 weeks old) received scopolamine (1 mg/kg, ip) 30 minutes before behavioral testing. Concurrently, mice were treated with Donepezil HCl (E2020) (1 mg/kg/day) dissolved in distilled water via oral gavage for 7 days. Morris water maze test was performed to evaluate spatial learning and memory [3] - Aβ-induced AD model: Male Sprague-Dawley rats (12 weeks old) were injected with Aβ₁₋₄₂ (10 μg) into the hippocampus. Seven days post-surgery, rats were given Donepezil HCl (E2020) (3 mg/kg/day, po) for 21 days. Passive avoidance test and hippocampal tissue analysis (Aβ deposition, ChAT activity) were conducted [3] - Aged rat memory impairment model: Male Sprague-Dawley rats (24 months old) received intraperitoneal injection of Donepezil HCl (E2020) (2 mg/kg) once daily for 14 days. Passive avoidance test was used to assess memory function [3] |
| 毒性/毒理 (Toxicokinetics/TK) |
In clinical use, Donepezil HCl (E2020) (5-10 mg/day, po) is associated with mild to moderate adverse events, including nausea (19%), diarrhea (15%), and insomnia (7%); no severe (hepatic/renal toxicity) is reported [3]
- Plasma protein binding of Donepezil HCl (E2020) is 96% in human plasma [3] - Acute oral LD50 of Donepezil HCl (E2020) in mice is 410 mg/kg, and 320 mg/kg in rats [3] |
| 参考文献 |
|
| 其他信息 |
Donepezil Hydrochloride is the hydrochloride salt of a piperidine derivative with neurocognitive-enhancing activity. Donepezil reversibly inhibits acetylcholinesterase, thereby blocking the hydrolysis of the neurotransmitter acetylcholine and, consequently, increasing its activity. This agent may improve neurocognitive function in Alzheimer's disease, reduce sedation associated with opioid treatment of cancer pain, and improve neurocognitive function in patients who have received radiation therapy for primary brain tumors or brain metastases.
An indan and piperidine derivative that acts as a selective and reversible inhibitor of ACETYLCHOLINESTERASE. Donepezil is highly selective for the central nervous system and is used in the management of mild to moderate DEMENTIA in ALZHEIMER DISEASE. See also: Donepezil (has active moiety); Donepezil hydrochloride; memantine hydrochloride (component of). Donepezil HCl (E2020) is a reversible, selective acetylcholinesterase (AChE) inhibitor with additional GSK-3 inhibitory activity [1,2] - Clinically approved indication is mild to moderate Alzheimer’s disease (AD), improving cognitive function by inhibiting AChE to increase synaptic acetylcholine levels in the brain [3] - Beyond cholinergic enhancement, the drug exerts neuroprotective effects by inhibiting GSK-3, reducing Aβ-induced neuronal apoptosis and promoting synaptic plasticity [2,3] - It has a long elimination half-life (70-80 hours in humans), allowing once-daily oral administration, which improves compliance in AD patients [3] |
| 分子式 |
C24H29NO3.HCL
|
|
|---|---|---|
| 分子量 |
416
|
|
| 精确质量 |
415.191
|
|
| 元素分析 |
C, 69.30; H, 7.27; Cl, 8.52; N, 3.37; O, 11.54
|
|
| CAS号 |
120011-70-3
|
|
| 相关CAS号 |
Donepezil;120014-06-4;Donepezil-d4 hydrochloride;1219798-88-5;Donepezil-d5 hydrochloride;1883548-90-0
|
|
| PubChem CID |
5741
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 沸点 |
527.9ºC at 760 mmHg
|
|
| 熔点 |
220-222ºC
|
|
| 闪点 |
273.1ºC
|
|
| 蒸汽压 |
3.11E-11mmHg at 25°C
|
|
| LogP |
5.101
|
|
| tPSA |
38.77
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
6
|
|
| 重原子数目 |
29
|
|
| 分子复杂度/Complexity |
510
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
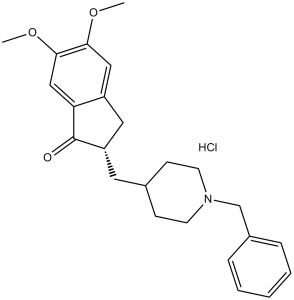
Cl[H].O=C1C2=C([H])C(=C(C([H])=C2C([H])([H])C1([H])C([H])([H])C1([H])C([H])([H])C([H])([H])N(C([H])([H])C2C([H])=C([H])C([H])=C([H])C=2[H])C([H])([H])C1([H])[H])OC([H])([H])[H])OC([H])([H])[H]
|
|
| InChi Key |
XWAIAVWHZJNZQQ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C24H29NO3.ClH/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18;/h3-7,14-15,17,20H,8-13,16H2,1-2H3;1H
|
|
| 化学名 |
2-((1-benzylpiperidin-4-yl)methyl)-5,6-dimethoxy-2,3-dihydro-1H-inden-1-one hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2933.39.9100
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.25 mg/mL (3.01 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 12.5 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1.25 mg/mL (3.01 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 12.5 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1.25 mg/mL (3.01 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 30% propylene glycol, 5% Tween 80, 65% D5W: 30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4038 mL | 12.0192 mL | 24.0385 mL | |
| 5 mM | 0.4808 mL | 2.4038 mL | 4.8077 mL | |
| 10 mM | 0.2404 mL | 1.2019 mL | 2.4038 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Clinical Trial to Access Pharmacokinetic Profiles and Safety of IVL3003.
CTID: NCT05345509
Phase: Phase 1/Phase 2 Status: Recruiting
Date: 2023-08-22
|
|