| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Vitamin D receptor
|
|---|---|
| 体外研究 (In Vitro) |
在接受肾切除术的小鼠中,多克骨化醇(100 或 300 皮克/克体重)可将血清钙和甲状旁腺激素 (PTH) 水平恢复至正常。在接受肾切除术的小鼠中,多克骨化醇(300 pg/g bw)可显着降低纤维性骨炎。在给予高盐 (HS) 饮食的大鼠中,多克骨化醇显着减少心脏肥大并增强心脏功能。在喂食高盐 (HS) 饮食的大鼠中,多克骨化醇治疗导致组织心房钠尿因子 (ANF) mRNA 水平和血浆脑钠尿肽 (BNP) 水平显着降低。此外,多克骨化醇可显着降低蛋白激酶 C-α (PKCα) 水平,表明维生素 D 缺乏与 PKC 介导的心脏肥大之间可能存在联系。在饮食诱导的肥胖 (DIO) 小鼠中,多克骨化醇可减少蛋白尿、足细胞损伤、系膜生长和细胞外基质蛋白积累。在 DIO 小鼠中,doxercalciferol 还可以减少促纤维化生长因子、促炎细胞因子、氧化应激和巨噬细胞浸润。此外,doxercalciferol 抑制 DIO 小鼠的肾素-血管紧张素-醛固酮系统激活,其中包括血管紧张素 II 1 型受体和盐皮质激素受体。在小鼠中,多克骨化醇和氯沙坦的组合最有效地预防白蛋白尿,恢复肾小球滤过屏障的结构,并以剂量依赖性方式显着降低肾小球硬化。当多克骨化醇和氯沙坦联合使用时,小鼠的糖尿病肾脏几乎没有表现出形态或分子变化。
|
| 体内研究 (In Vivo) |
在 5/6 肾切除 (NX) 大鼠中,第 6 周时,多克骨化醇(0.083、0.167 或 0.333 μg/kg,腹腔注射)可升高血清磷。此外,多克骨化醇(0.167 和 0.333 μg/kg)可增强脉搏波速度 (PWV) 的增加)在第 6 周的 5/6 肾切除 (NX) 大鼠中,并在第 2 周和第 6 周升高血清钙和 Ca × P。多克骨化醇将血清 PTH 降低至 SHAM 水平,并防止 PTH 上升至 0.083 μg/kg[1]。在饲喂高脂肪饮食的 NON 小鼠中,多克骨化醇(125 ng/kg,腹腔注射,每周 3 次)增加 VDR mRNA 水平的表达和 TRPV5 的肾表达。在接受 HF 饮食的小鼠中,多克骨化醇还可以减少蛋白尿,阻止足细胞损失,并减少细胞外基质蛋白的积累。在饲喂 HF 饮食的小鼠中,多克骨化醇可阻断肾素-血管紧张素-醛固酮系统表达的增加,并抑制促纤维化生长因子(TGF-β、PAI-1 和结缔组织生长因子 (CTGF))的表达。此外,Doxercalciferol 还可抑制巨噬细胞的浸润,降低 NF-κb 活性,停止促炎细胞因子的表达,并阻止高脂饮食小鼠肾脂质的积累[2]。当对链脲佐菌素诱导的糖尿病小鼠每周 3 次腹膜内 (ip) 给药时,多克骨化醇 (30 ng/kg) 显着减轻足细胞损失和细胞凋亡,并减少肾小球纤维化[3]。
|
| 动物实验 |
Rats: One week following nephrectomy, male Sprague-Dawley 5/6 nephrectomized (NX) rats (∼200 mg) are used. A typical surgical ablation procedure consisting of two steps is used to perform the nephrectomy. Rats are kept on a high-phosphorus diet (0.9% phosphorus and 0.6% calcium) for the duration of the study starting two weeks after nephrectomy in order to cause secondary hyperparathyroidism. Day 0: Vehicle (5% EtOH/95% propylene glycol; 0.4 mL/kg; i.p.) or VDRA (paricalcitol or Doxercalciferol; 0.083, 0.167, or 0.333 μg/kg; intraperitoneally) is given three times a week for 41 days (n = 6–10 per group) to SHAM and 5/6 NX rats (n = 7–10 per group). These dosages were selected because, in this CKD model, after two or six weeks of treatment, lower doses (0.021 and 0.042 μg/kg; i.p.) of either compound do not suppress PTH. Days 0 through 41 are when blood is drawn (24 hours after the dose). Animals are given ketamine (50 mg/kg) anesthesia on Days 0, 13, and 41 (24 h post-dose), and blood is drawn from the tail vein for measurements of PTH and serum blood chemistry[1].
|
| 药代性质 (ADME/PK) |
Metabolism / Metabolites
Doxercalciferol is absorbed from the gastrointestinal tract and activated by CYP 27 in the liver to form 1α,25-(OH)2D2 (major metabolite) and 1α,24-dihydroxyvitamin D2 (minor metabolite). Activation of doxercalciferol does not require the involvement of the kidneys. Biological Half-Life 32 to 37 hours. |
| 参考文献 |
|
| 其他信息 |
Doxercalciferol is a hydroxy seco-steroid and synthetic vitamin D2 analogue that undergoes metabolic activation in vivo to form 1alpha,25-dihydroxyvitamin D2 (1alpha,25-(OH)2D2), a naturally occurring, biologically active form of vitamin D2. It is used to treat secondary hyperparathyroidism, a condition in which the body produces excess parathyroid hormone (PTH; a natural substance needed to control the amount of calcium in the blood) in certain people with chronic kidney disease. It has a role as a provitamin, a bone density conservation agent and a prohormone. It is a vitamin D and a hydroxy seco-steroid.
Doxercalciferol is a synthetic vitamin D2 analog that undergoes metabolic activation in vivo to form 1α,25-dihydroxyvitamin D2 (1α,25-(OH)2D2), a naturally occurring, biologically active form of vitamin D2. Doxercalciferol is indicated for the treatment of secondary hyperparathyroidism in patients with chronic kidney disease on dialysis, as well as for the treatment of secondary hyperparathyroidism in patients with Stage 3 or Stage 4 chronic kidney disease. Doxercalciferol is marketed under the brand name Hectoral by Genzyme Corporation, and is manufactured by Catalent Pharma Solutions, Inc. Doxercalciferol is a Vitamin D2 Analog. Doxercalciferol is a synthetic analog of vitamin D with potential antineoplastic activity. In the liver, doxercalciferol is converted to its biologically active vitamin D metabolites, which control the intestinal absorption of dietary calcium, the tubular reabsorption of calcium by the kidney and, in conjunction with parathyroid hormone (PTH), the mobilization of calcium from the skeleton. Through interaction with specific receptor proteins in target tissues, these vitamin D metabolites act directly on osteoblasts to stimulate skeletal growth, and on the parathyroid glands to suppress PTH synthesis and secretion. This agent has also been shown to inhibit the growth of retinoblastomas, and may exhibit some antiproliferative activity against prostate cancer cells. Drug Indication Doxercalciferol is indicated for the treatment of secondary hyperparathyroidism in patients with chronic kidney disease on dialysis, as well as for the treatment of secondary hyperparathyroidism in patients with Stage 3 or Stage 4 chronic kidney disease. FDA Label Mechanism of Action Calcitriol (1α,25-(OH)2D3) and 1α,25-(OH)2D2 regulate blood calcium at levels required for essential body functions. Specifically, the biologically active vitamin D metabolites control the intestinal absorption of dietary calcium, the tubular reabsorption of calcium by the kidney and, in conjunction with parathyroid hormone (PTH), the mobilization of calcium from the skeleton. They act directly on bone cells (osteoblasts) to stimulate skeletal growth, and on the parathyroid glands to suppress PTH (parathyroid hormone) synthesis and secretion. These functions are mediated by the interaction of these biologically active metabolites with specific receptor proteins in the various target tissues. In patients with chronic kidney disease (CKD), deficient production of biologically active vitamin D metabolites (due to lack of or insufficient 25-hydroxyvitamin D-1-alpha-hydroxylase activity) leads to secondary hyperparathyroidism, which contributes to the development of metabolic bone disease. |
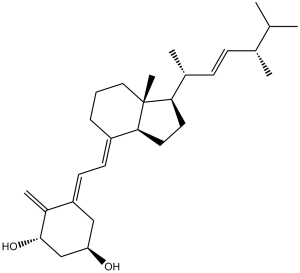
| 分子式 |
C28H44O2
|
|
|---|---|---|
| 分子量 |
412.65
|
|
| 精确质量 |
412.334
|
|
| 元素分析 |
C, 81.50; H, 10.75; O, 7.75
|
|
| CAS号 |
54573-75-0
|
|
| 相关CAS号 |
trans-Doxercalciferol;74007-20-8;Impurity of Doxercalciferol;127516-23-8
|
|
| PubChem CID |
5281107
|
|
| 外观&性状 |
White to yellow/brown solid powder
|
|
| 密度 |
1.0±0.1 g/cm3
|
|
| 沸点 |
538.7±50.0 °C at 760 mmHg
|
|
| 熔点 |
138-140ºC
|
|
| 闪点 |
224.0±24.7 °C
|
|
| 蒸汽压 |
0.0±3.3 mmHg at 25°C
|
|
| 折射率 |
1.541
|
|
| LogP |
8.15
|
|
| tPSA |
40.46
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
2
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
30
|
|
| 分子复杂度/Complexity |
712
|
|
| 定义原子立体中心数目 |
7
|
|
| SMILES |
C=C1[C@H](C[C@@H](C/C1=C/C=C2[C@]3([C@@](C)([C@H](CC3)[C@@H](/C=C/[C@@H](C(C)C)C)C)CCC/2)[H])O)O
|
|
| InChi Key |
HKXBNHCUPKIYDM-CGMHZMFXSA-N
|
|
| InChi Code |
InChI=1S/C28H44O2/c1-18(2)19(3)9-10-20(4)25-13-14-26-22(8-7-15-28(25,26)6)11-12-23-16-24(29)17-27(30)21(23)5/h9-12,18-20,24-27,29-30H,5,7-8,13-17H2,1-4,6H3/b10-9+,22-11+,23-12-/t19-,20+,24+,25+,26-,27-,28+/m0/s1
|
|
| 化学名 |
(1R,3S,5Z)-5-[(2E)-2-[(1R,3aS,7aR)-1-[(E,2R,5R)-5,6-dimethylhept-3-en-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylidenecyclohexane-1,3-diol
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: (1). 本产品在运输和储存过程中需避光。 (2). 该产品在溶液状态不稳定,请现配现用。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.06 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.06 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4234 mL | 12.1168 mL | 24.2336 mL | |
| 5 mM | 0.4847 mL | 2.4234 mL | 4.8467 mL | |
| 10 mM | 0.2423 mL | 1.2117 mL | 2.4234 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02859896 | Active Recruiting |
Drug: Doxercalciferol (GZ427397) Drug: Calcitriol |
Secondary Hyperparathyroidism- Chronic Kidney Disease |
Sanofi | January 19, 2017 | Phase 3 |
| NCT00889629 | Completed | Drug: Doxercalciferol Drug: placebo |
Chronic Kidney Disease Kidney Transplantation |
Mariana Markell | November 2008 | Phase 4 |
| NCT00285467 | Completed | Drug: doxercalciferol Drug: Cholecalciferol |
Renal Osteodystrophy | Indiana University School of Medicine |
January 2006 | Not Applicable |
| NCT02282813 | Completed | Drug: Doxercalciferol Drug: Calcitriol |
Chronic Kidney Disease Vitamin D Deficiency |
OPKO Health, Inc. | April 2013 | Phase 3 |
| NCT00792857 | Completed | Drug: CTAP201 Injection Drug: Doxercalciferol |
Chronic Kidney Disease Chronic Renal Failure |
OPKO IP Holdings II, Inc. | November 2008 | Phase 1 |
|
|
|
|
|