| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
在人心房肌细胞膜片钳研究中,无人机成功抑制了峰值钠电流,在 3 μM 浓度下实现了 97% 的阻断 [1]。无人机抑制豚鼠心室肌细胞延迟整流钾电流:内向整流钾电流(IC50>30 μM)、缓慢激活的延迟整流钾电流(IC50=10 μM)、快速激活的延迟整流钾电流(IC50< 3μM)。 L型钙电流(IC50=0.18 μM)[1]也是如此。在兔心房结细胞 (IC50=63 nM) 和豚鼠心房细胞 (IC50=10 nM) 中,决奈达隆表现出对乙酰胆碱激活钾电流 (IK-Ach) 的有效抑制作用。决奈达隆阻断 IK-Ach 的效果是胺碘酮的 100 倍 [1]。决奈达隆以非竞争性方式与 β-肾上腺素能受体结合 (IC50=1.8 μM),通过阻止激动剂引起的腺苷酸环化酶活性升高来产生抗肾上腺素能作用 [1]。在离体豚鼠心脏中,达罗达隆 (0.01-1 μM) 剂量可诱导冠状动脉灌注压呈剂量依赖性降低。其钙电流阻断可能与该作用有关,该作用不依赖于一氧化氮合酶途径[1]。
|
|---|---|
| 体内研究 (In Vivo) |
决奈达隆(腹膜内注射;25-100 mg/kg)显示剂量依赖性抗惊厥作用并提高小鼠电惊厥阈值[2]。
|
| 动物实验 |
Animal/Disease Models: Tonic-clonic seizures in male albino Swiss outbred mice [2]
Doses: 25 mg/kg; 50 mg/kg; 75 mg/kg; 100 mg/kg Route of Administration: intraperitoneal (ip) injection Experimental Results: Shown Produces significant anticonvulsant effects. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Dronedarone is well absorbed after oral administration (>70%). It displays low systemic bioavailability due to extensive first-pass metabolism. The absolute bioavailability of dronedarone without and with a high-fat meal is 4% and 15%, respectively. The peak plasma concentrations of dronedarone and its main circulating N-debutyl metabolite are reached within 3 to 6 hours after administration with food. Following repeated administration of 400 mg dronedarone twice daily, the steady-state was reached within 4 to 8 days of initial treatment. The steady-state Cmax and systemic exposure to the N-debutyl metabolite are similar to that of the parent compound. Following oral administration, about 84% of the labeled dose is excreted in feces and 6% is excreted in urine, mainly as metabolites. Unchanged parent compound and the N-debutyl metabolite accounted for less than 15% of the total radioactivity in the plasma. The volume of distribution at steady-state ranges from 1200 to 1400 L following intravenous administration. Following intravenous administration, the clearance ranged from 130 to 150 L/h. The in vitro plasma protein binding of dronedarone and its N-debutyl metabolite is 99.7% and 98.5% respectively and is not saturable. Both compounds bind mainly to albumin. After intravenous administration the volume of distribution at steady state (Vss) ranges from 1,200 to 1,400 L. Following oral administration in fed condition, dronedarone is well absorbed (at least 70%). However due to presystemic first pass metabolism, the absolute bioavailability of dronedarone (given with food) is 15%. Concomitant intake of food increases dronedarone bioavailability by on average 2- to 4- fold. After oral administration in fed conditions, peak plasma concentrations of dronedarone and the main circulating active metabolite (N-debutyl metabolite) are reached within 3 to 6 hours. After repeated administration of 400 mg twice daily, steady state is reached within 4 to 8 days of treatment and the mean accumulation ratio for dronedarone ranges from 2.6 to 4.5. The steady state mean dronedarone Cmax is 84-147 ng/mL and the exposure of the main N-debutyl metabolite is similar to that of the parent compound. The pharmacokinetics of dronedarone and its N-debutyl metabolite both deviate moderately from dose proportionality: a 2-fold increase in dose results in an approximate 2.5- to 3.0-fold increase with respect to Cmax and AUC. After oral administration, approximately 6% of the labelled dose is excreted in urine mainly as metabolites (no unchanged compound excreted in urine) and 84% are excreted in feces mainly as metabolites. After IV administration the plasma clearance of dronedarone ranges from 130 to 150 l/h. The terminal elimination half-life of dronedarone is around 25-30 hours and that of its N-debutyl metabolite around 20-25 hours. In patients, dronedarone and its metabolite are completely eliminated from the plasma within 2 weeks after the end of a 400 mg twice daily- treatment. Metabolism / Metabolites Dronedarone predominantly undergoes CYP3A-mediated hepatic metabolism. Initial metabolism of dronedarone involves N-debutylation to form the N-debutyl-dronedarone, which retains 1/10 to 1/3 of pharmacological activity of the parent compound. N-debutyl-dronedarone can be further metabolized to phenol-dronedarone via O-dealkylation and propanoic acid-dronedarone via oxidative deamination. Dronedarone can also be metabolized by CYP2D6 to form benzofuran-hydroxyl-dronedarone. Other detectable metabolites include C-dealkyl-dronedarone and dibutylamine-hydroxyl-dronedarone, along with other minor downstream metabolites with undetermined chemical structures. Dronedarone is extensively metabolised, mainly by CYP 3A4 (see section 4.5). The major metabolic pathway includes N-debutylation to form the main circulating active metabolite followed by oxidation, oxidative deamination to form the inactive propanoic acid metabolite, followed by oxidation, and direct oxidation. The N-debutyl metabolite exhibits pharmacodynamic activity but is 3 to 10-times less potent than dronedarone. This metabolite contributes to the pharmacological activity of dronedarone in humans. Biological Half-Life The elimination half life ranges from 13 to 19 hours. The terminal elimination half-life of dronedarone is around 25-30 hours and that of its N-debutyl metabolite around 20-25 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Chronic therapy with dronedarone has been associated with mild serum enzyme elevations in up to 12% of patients, but similar rates were found in comparator arms and even in placebo recipients. The serum aminotransferase elevations that occur during chronic dronedarone therapy are generally mild-to-moderate in severity and asymptomatic, rarely requiring discontinuation or dose modification. In preapproval clinical trials, clinically apparent liver injury was not described. Since its approval and more wide scale use, however, dronedarone has been linked to several cases of clinically apparent liver injury with jaundice, some of which have been severe. The onset of injury ranged from 2 to 11 months and the clinical presentation was similar to acute viral hepatitis, with symptoms of fatigue and abdominal discomfort followed by jaundice and a hepatocellular pattern of serum enzyme elevations. Several instances have resulted in acute liver failure requiring emergency liver transplantation. However, specific clinical features of cases of clinically apparent liver injury from dronedarone have not been well defined and the relationship of dronedarone to the described liver injury has not always been well documented. Likelihood score: C (probable cause of clinically apparent liver injury). Protein Binding The _in vitro_ plasma protein binding of dronedarone and its N-debutyl metabolite is 99.7% and 98.5%, respectively. Both mainly bind to albumin and are not capable of saturation. Interactions Dronedarone can increase plasma concentrations of tacrolimus, sirolimus, and other CYP 3A substrates with a narrow therapeutic range when given orally. Monitor plasma concentrations and adjust dosage appropriately. Dronedarone increased simvastatin/simvastatin acid exposure by 4- and 2-fold, respectively. Because of multiple mechanisms of interaction with statins (CYPs and transporters), follow statin label recommendations for use with CYP 3A and P-gP inhibitors such as dronedarone. Pantoprazole, a drug that increases gastric pH, did not have a significant effect on dronedarone pharmacokinetics. Verapamil and diltiazem are moderate CYP 3A inhibitors and increase dronedarone exposure by approximately 1.4-to 1.7-fold. For more Interactions (Complete) data for Dronedarone (16 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Antiarrhythmic Multaq is indicated to reduce the risk of cardiovascular hospitalization in patients with paroxysmal or persistent atrial fibrillation (AF) or atrial flutter (AFL), with a recent episode of AF/AFL and associated cardiovascular risk factors (i.e., age greaer than 70, hypertension, diabetes, prior cerebrovascular accident, left atrial diameter greater than or equal to 50 mm or left ventricular ejection fraction (LVEF) less than 40%), who are in sinus rhythm or who will be cardioverted. /Included in US product label/ Drug Warnings /BOXED WARNING/ WARNING: INCREASED RISK OF DEATH, STROKE AND HEART FAILURE IN PATIENTS WITH DECOMPENSATED HEART FAILURE OR PERMANENT ATRIAL FIBRILLATION. In patients with symptomatic heart failure and recent decompensation requiring hospitalization or NYHA Class IV heart failure; Multaq doubles the risk of death. Multaq is contraindicated in patients with symptomatic heart failure with recent decompensation requiring hospitalization or NYHA Class IV heart failure. In patients with permanent atrial fibrillation, Multaq doubles the risk of death, stroke and hospitalization for heart failure. Multaq is contraindicated in patients in atrial fibrillation (AF) who will not or cannot be cardioverted into normal sinus rhythm. Multaq is contraindicated in patients with New York Heart Association (NYHA) Class IV heart failure, or NYHA Class II - III heart failure with a recent decompensation requiring hospitalization or referral to a specialized heart failure clinic. In a placebo-controlled study in patients with severe heart failure requiring recent hospitalization or referral to a specialized heart failure clinic for worsening symptoms (the ANDROMEDA Study), patients given dronedarone had a greater than two-fold increase in mortality. Such patients should not be given dronedarone Postmarketing cases of new onset and worsening heart failure have been reported during treatment with Multaq. Advise patients to consult a physician if they develop signs or symptoms of heart failure, such as weight gain, dependent edema, or increasing shortness of breath. If heart failure develops or worsens, consider the suspension or discontinuation of Multaq. Hepatocellular liver injury, including acute liver failure requiring transplant, has been reported in patients treated with multaq in the post-marketing setting. Advise patients treated with Multaq to report immediately symptoms suggesting hepatic injury (such as anorexia, nausea, vomiting, fever, malaise, fatigue, right upper quadrant pain, jaundice, dark urine, or itching). Consider obtaining periodic hepatic serum enzymes, especially during the first 6 months of treatment. It is not known whether routine periodic monitoring of serum enzymes will prevent the development of severe liver injury. If hepatic injury is suspected, promptly discontinue Multaq and test serum enzymes, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase, as well as serum bilirubin, to establish whether there is liver injury. If liver injury is found, institute appropriate treatment and investigate the probable cause. Do not restart Multaq in patients without another explanation for the observed liver injury. For more Drug Warnings (Complete) data for Dronedarone (18 total), please visit the HSDB record page. Pharmacodynamics Dronedarone is an antiarrhythmic agent that restores normal sinus rhythm and reduces heart rate in atrial fibrillation. In another model, it prevents ventricular tachycardia and ventricular fibrillation. Dronedarone moderately prolongs the QTc interval by about 10 ms on average. Dronedarone decreases arterial blood pressure and reduces oxygen consumption. It reduces myocardial contractility with no change in left ventricular ejection fraction. Dronedarone vasodilates coronary arteries through activation of the nitric oxide pathway. In clinical studies, dronedarone reduced incidence of hospitalizations for acute coronary syndromes and reduced incidence of stroke. Dronedarone exhibits antiadrenergic effects by reducing alpha-adrenergic blood pressure response to epinephrine and beta 1 and beta 2 responses to isoproterenol. Dronedarone was shown to inhibit triiodothyronine (T3) signalling by binding to TRα1 but much less so to TRβ1. The treatment of dronedarone in patients with severe heart failure and left ventricular systolic dysfunction was associated with increased early mortality related to the worsening of heart failure. In animal studies, the use of dronedarone at doses equivalent to the recommended human doses was associated with fetal harm. In clinical studies and postmarketing reports, dronedarone was shown to cause hepatocellular liver injury and pulmonary toxicities, such as interstitial lung disease, pneumonitis, and pulmonary fibrosis. Compared to its related compound [amiodarone], dronedarone has a faster onset and offset of actions with a shorter elimination half-life and low tissue accumulation. |
| 分子式 |
C31H44N2O5S
|
|---|---|
| 分子量 |
556.76
|
| 精确质量 |
556.297
|
| CAS号 |
141626-36-0
|
| 相关CAS号 |
Dronedarone Hydrochloride;141625-93-6;Dronedarone-d6 hydrochloride;1329809-23-5
|
| PubChem CID |
208898
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
683.9±65.0 °C at 760 mmHg
|
| 熔点 |
137-145
149-153 °C |
| 闪点 |
367.4±34.3 °C
|
| 蒸汽压 |
0.0±2.1 mmHg at 25°C
|
| 折射率 |
1.564
|
| LogP |
7.58
|
| tPSA |
97.23
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
18
|
| 重原子数目 |
39
|
| 分子复杂度/Complexity |
800
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
ZQTNQVWKHCQYLQ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C31H44N2O5S/c1-5-8-12-29-30(27-23-25(32-39(4,35)36)15-18-28(27)38-29)31(34)24-13-16-26(17-14-24)37-22-11-21-33(19-9-6-2)20-10-7-3/h13-18,23,32H,5-12,19-22H2,1-4H3
|
| 化学名 |
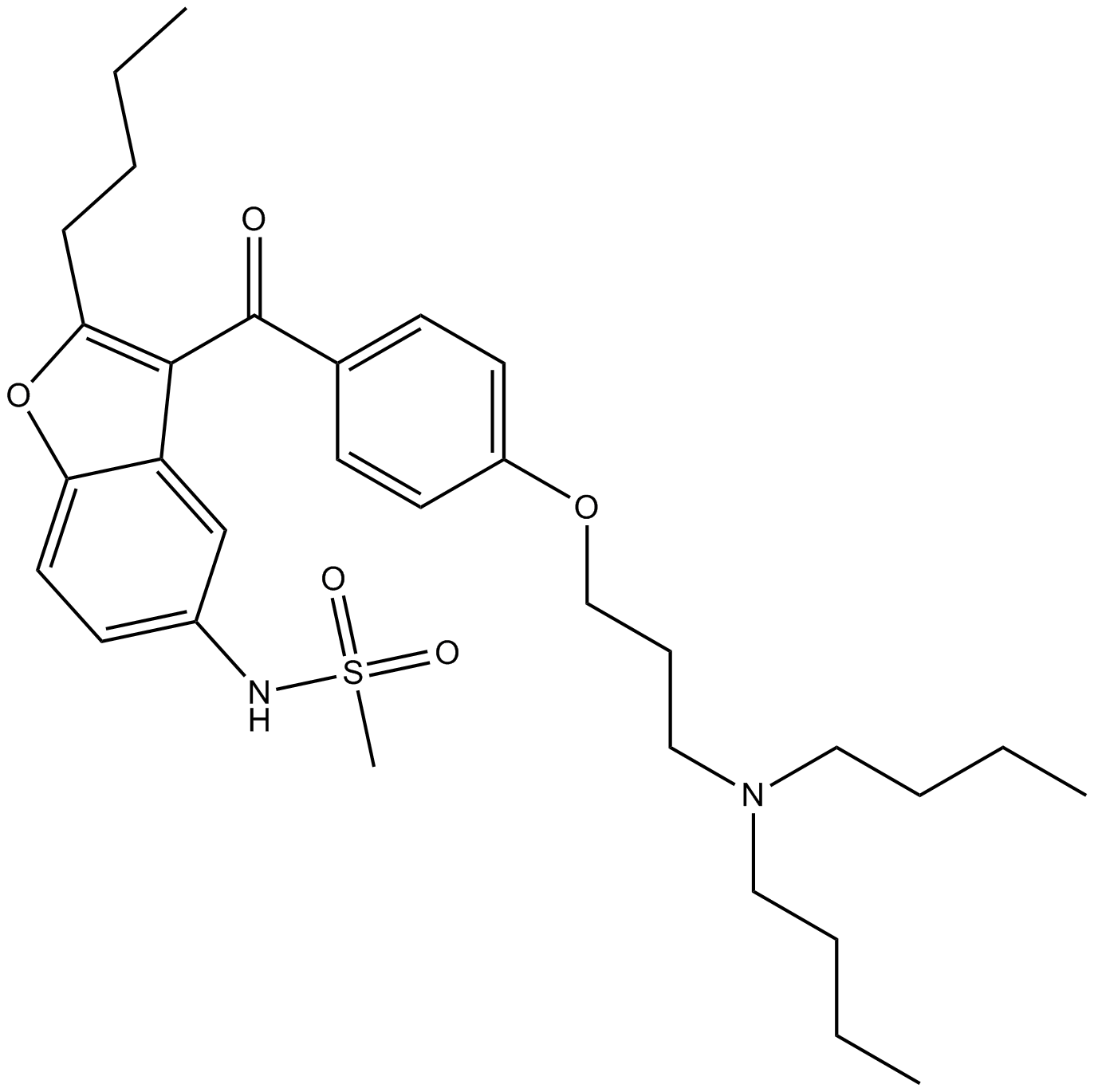
N-[2-butyl-3-[4-[3-(dibutylamino)propoxy]benzoyl]-1-benzofuran-5-yl]methanesulfonamide
|
| 别名 |
SR 33589 SR33589D03914 S7529D4689 W3083 RL01735D-03914 S-7529D-4689 W-3083 RL-01735Multaq
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~89.81 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.49 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.49 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.49 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 悬浮液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7961 mL | 8.9805 mL | 17.9611 mL | |
| 5 mM | 0.3592 mL | 1.7961 mL | 3.5922 mL | |
| 10 mM | 0.1796 mL | 0.8981 mL | 1.7961 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Prophylaxis Against Postoperative Atrial Fibrillation in Patients Undergoing On-pump CABG
CTID: NCT03905759
Phase: Phase
Dronedarone in pacemaker patients with paroxysmal atrial fibrillation
CTID: null
Phase: Phase 4 Status: GB - no longer in EU/EEA
Date: 2010-07-02