| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
progestogen Receptor
|
|---|---|
| 体外研究 (In Vitro) |
屈螺酮(10-150 μM,24-48 小时)对 PLHC-1 细胞的毒性作用的有效浓度限值(EC50 值)为 105-119 μM(持续 24 小时)和 51-58 μM(持续 48 小时)[1]。 Drospirenone(0.01-10 µM,24 小时)可抑制 HEEC 水溶液中的 PAI-1 和 tPA [2]。 PLHC-1 中细胞 ROS 在 -200 µM 下生成 15-120 分钟 [2]。在小鼠模型 S9 组分中添加 100 µM(72 小时)可能会损坏 MCF-7 细胞中的 DNA [3]。
|
| 体内研究 (In Vivo) |
在雄性和雌性小鼠中,连续五天口服多螺酮(10-100 mg/kg)会损害骨髓细胞中的 DNA [3]。
|
| 细胞实验 |
细胞毒性测定 [1]
细胞类型: PLHC-1 细胞 测试浓度: 10-150 µM 孵育时间: 24 hrs(小时)、48 hrs(小时) 实验结果:细胞活力降低50%,24 hrs(小时后有效浓度)浓度为102-119μM,48小时后有效浓度为51-58μM。 |
| 动物实验 |
Animal/Disease Models: Adult female mice [3]
Doses: 10 mg/kg, 100mg/kg Route of Administration: po (oral gavage) Experimental Results: DNA damage was induced at dose rates of 10 and 100 mg/kg. Concomitant use with ethinyl estradiol enhances genotoxicity. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The absolute bioavailability of drospirenone is approximately 76% due to first-pass effects. The maximum plasma concentration of drospirenone occurs within 1 to 2 hours after oral administration and is estimated to range between 60 and 87 ng/mL. A European prescribing monograph for the combination product of estradiol and drospirenone indicates that drospirenone is both completely and rapidly absorbed. It reports a Cmax of 21.9 ng/ml, achieved approximately 1-hour post-administration. The absolute bioavailability is reported to range between 76 to 85%. Various metabolites of drospirenone are measured in the urine and feces. Drospirenone elimination from the body is almost after 10 days post-administration when negligible amounts of drospirenone are found unchanged in both the urine and feces. Between 38% to 47% of the metabolites are identified as glucuronide and sulfate conjugates in the urine. In the feces, approximately 17% to 20% of identifiable metabolites are found to be excreted as glucuronides and sulfates. The volume of distribution of drospirenone is estimated to be 4 L/kg, according to the FDA label for Yaz. Prescribing information from a combination of estradiol and drospirenone estimates the volume of distribution to range from 3.7- 4.2 L/kg. Drospirenone is rapidly cleared, typically within 2-3 days of administration of the last active tablet. The rate of clearance of drospirenone calculated in the serum ranges from 1.2-1.5 ml/min/kg, however, this value can vary by up to 25% according to the patient. The absolute bioavailability of drospirenone (DRSP) from a single entity tablet is about 76%. Serum concentrations of DRSP and EE reached peak levels within 1-2 hours after administration of Gianvi. The pharmacokinetics of DRSP are dose proportional following single doses ranging from 1-10 mg. Following daily dosing of Gianvi, steady state DRSP concentrations were observed after 8 days. There was about 2 to 3 fold accumulation in serum Cmax and AUC (0-24hr) values of DRSP following multiple dose administration of Gianvi. The rate of absorption of DRSP and EE following single administration of a formulation similar to Gianvi was slower under fed (high fat meal) conditions with the serum Cmax being reduced about 40% for both components. The extent of absorption of DRSP, however, remained unchanged. DRSP and EE serum levels decline in two phases. The apparent volume of distribution of DRSP is approximately 4 L/kg and that of EE is reported to be approximately 4-5 L/kg. For more Absorption, Distribution and Excretion (Complete) data for Drospirenone (10 total), please visit the HSDB record page. Metabolism / Metabolites Drospirenone is heavily metabolized. The two major inactive metabolites identified are the acid form of drospirenone produced by the opening of its lactone ring, known as M11, and the 4,5-dihydro-drospirenone-3-sulfate (M14). Drospirenone also undergoes oxidative metabolism via the hepatic cytochrome enzyme CYP3A4. The two main metabolites of DRSP found in human plasma were identified to be the acid form of DRSP generated by opening of the lactone ring and the 4,5-dihydrodrospirenone-3-sulfate. These metabolites were shown not to be pharmacologically active. In in vitro studies with human liver microsomes, DRSP was metabolized only to a minor extent mainly by Cytochrome P450 3A4 (CYP3A4). Biological Half-Life The serum half-life of drospirenone is estimated to be 30 hours. The half-life of drospirenone metabolite excretion in the urine and feces is approximately 40 hours. DRSP serum levels are characterized by a terminal disposition phase half-life of approximately 30 hours after both single and multiple dose regimens. |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Drospirenone is about 95% to 97% bound to serum plasma protein, likely to albumin. During in vitro studies, drospirenone was found to bind with low affinity to sex hormone-binding globulin (SHBG). Another reference indicates that drospirenone binds to serum albumin but does not bind to sex hormone-binding globulin (SHBG), nor corticoid binding globulin (CBG). Only 3-5% of the total drospirenone concentration is measured as a free steroid. |
| 参考文献 |
|
| 其他信息 |
Drospirenone is a steroid lactone and a 3-oxo-Delta(4) steroid. It has a role as a contraceptive drug, an aldosterone antagonist and a progestin.
Drospirenone is a synthetic progestin commonly found in the popular oral contraceptive, Yaz in combination with [Ethinyl estradiol]. Most recently, it was approved by both Health Canada and the FDA in combination with [Estetrol] as an oral contraceptive therapy. Aside from its contraceptive effects, drospirenone is used with estrogens to control acne and premenstrual dysphoric disorder (PMDD). Drospirenone has been the subject of widespread safety concern due to the possibility of an increased risk of venous thromboembolism associated with its use. In 2012, however, a safety statement by the FDA concluded that the increase in the risk of thromboembolism resulting from the use of drospirenone remains unclear, as studies regarding this risk are conflicting. Some studies have demonstrated a significantly increased risk and some demonstrating no risk of thromboembolic events. In its statement, the FDA has mentioned that increased risk of venous thromboembolism with oral contraceptives such as drospirenone exists but remains lower than the risk of this condition during pregnancy and during the postpartum period, and this should be considered when assessing potential risks of hormonal contraceptive use. Drospirenone is a Progestin. Drospirenone has been reported in Macaca fascicularis with data available. Drospirenone is a synthetic spironolactone analogue and progestin with progestational and anti-mineralocorticoid activity. Drospirenone binds to the progesterone receptor, the resulting complex becomes activated and binds to specific sites on DNA. This results in a suppression of LH activity and an inhibition of ovulation as well as an alteration in the cervical mucus and endometrium. This leads to an increased difficulty of sperm entry into the uterus and implantation. This drug is used in oral contraceptives. See also: Drospirenone; estradiol (component of); Drospirenone; estetrol (component of) ... View More ... Drug Indication Drospirenone, in combination with ethinyl estradiol or estetrol, is indicated as an oral contraceptive for the prevention of pregnancy. In addition to its use for contraceptive effects, this combination is used to treat moderate acne vulgaris and the symptoms of premenstrual dysphoric disorder. The drug has approved indications for combination with estrogens for the treatment of menopause-associated symptoms, such as vasomotor symptoms and vulvovaginal atrophy. Drospirenone combined with estrogen may also may aid in the prevention of osteoporosis in women who have been post-menopausal for at least a year and are not candidates for other therapies. It can sometimes be found in preparations containing estrogen and folic acid for folic acid replenishment during oral contraception. When used for the treatment of acne vulgaris, drospirenone-containing contraceptives should only be used in women ≥14 years of age who have experienced menarche, desire oral contraception, and do not have any contraindications to oral contraceptives. Off-label uses for this drug include the treatment of menstrual irregularities, dysmenorrhea, hirsutism, and endometriosis. FDA Label Treatment of endometriosis Prevention of pregnancy Mechanism of Action Drospirenone and ethinyl estradiol in combination suppress the release of follicle stimulating hormone (FSH) and luteinizing hormone (LH), preventing ovulation. Other changes induced by this drug which may aid in the prevention of pregnancy include alterations in cervical mucus consistency, hindering sperm movement, and lowering the chance of embryo implantation. Drospirenone is an analog of the diuretic spironolactone, which exerts anti-mineralocorticoid activity, blocking aldosterone receptors, which increases sodium and water excretion. Studies in animals have demonstrated that drospirenone administration leads to antiandrogenic activity. This activity helps to oppose the effects of naturally occurring androgens, inhibiting the binding of dihydrotestosterone (DHT) to its receptor, and preventing androgen synthesis in the ovaries, helping to treat acne and hirsutism. Drospirenone may also decrease the level of edema in sebaceous follicle during the second half of the menstrual cycle, when acne often appears. Combination oral contraceptives (COCs) act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increases the difficulty of sperm entry into the uterus) and the endometrium (which reduces the likelihood of implantation). Drospirenone is a spironolactone analogue with antimineralocorticoid activity. Preclinical studies in animals and in vitro have shown that drospirenone has no androgenic, estrogenic, glucocorticoid, or antiglucocorticoid activity. Preclinical studies in animals have also shown that drospirenone has antiandrogenic activity. Acne vulgaris is a skin condition with a multifactorial etiology including androgen stimulation of sebum production. While the combination of ethinyl estradiol and drospirenone increases sex hormone binding globulin (SHBG) and decreases free testosterone, the relationship between these changes and a decrease in the severity of facial acne in otherwise healthy women with this skin condition has not been established. The impact of the antiandrogenic activity of drospirenone on acne is not known. .... The pharmacological properties of drospirenone were investigated in vitro by receptor binding and transactivation experiments and in vivo in appropriate animal models. In qualitative agreement with progesterone, the compound binds strongly to the progesterone and the mineralocorticoid receptor and with lower affinity to androgen and glucocorticoid receptors. There is no detectable binding to the estrogen receptor. Steroid hormone agonistic and antagonistic activities of progesterone and drospirenone were compared in transactivation experiments. Individual steroid hormone receptors were artificially expressed together with a reporter gene in appropriate cell lines. Both hormones were unable to induce any androgen receptor-mediated agonistic activity. Rather, both progesterone and drospirenone distinctly antagonized androgen-stimulated transcriptional activation. Likewise, both compounds only very weakly activated the mineralocorticoid receptor but showed potent aldosterone antagonistic activity. Drospirenone did not induce glucocorticoid receptor-driven transactivation. Progesterone was a weak agonist in this respect. Drospirenone exerts potent progestogenic and antigonadotropic activity which was studied in various animal species. It efficiently promotes the maintenance of pregnancy in ovariectomized rats, inhibits ovulation in rats and mice and stimulates endometrial transformation in the rabbit. Furthermore, drospirenone shows potent antigonadotropic, i.e., testosterone-lowering activity in male cynomolgus monkeys. The progestogenic potency of drospirenone was found to be in the range of that of norethisterone acetate. The majority of clinically used progestogens are androgenic. Drospirenone, like progesterone, has no androgenic but rather an antiandrogenic effect. This property was demonstrated in castrated, testosterone propionate substituted male rats by a dose-dependent inhibition of accessory sex organ growth (seminal vesicles, prostate). In this model, the potency of drospirenone was about a third that of cyproterone acetate. Drospirenone, like progesterone, shows antimineralocorticoid activity, which causes moderately increased sodium and water excretion. This is an outstanding characteristic which has not been described for any other synthetic progestogen before. Drospirenone is eight to ten times more effective in this respect than spironolactone. The natriuretic effect was demonstrable for at least three weeks upon daily treatment of rats with a dose of 10 mg/animal. Drospirenone is devoid of any estrogenic, glucocorticoid or antiglucocorticoid activity. In summary, drospirenone, like progesterone, combines potent progestogenic with antimineralocorticoid and antiandrogenic activity in a similar dose range. For more Mechanism of Action (Complete) data for Drospirenone (9 total), please visit the HSDB record page. |
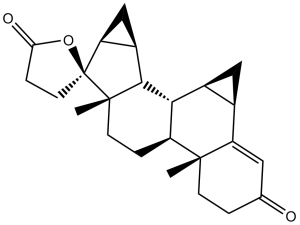
| 分子式 |
C24H30O3
|
|---|---|
| 分子量 |
366.49
|
| 精确质量 |
366.219
|
| 元素分析 |
C, 78.65; H, 8.25; O, 13.10
|
| CAS号 |
67392-87-4
|
| 相关CAS号 |
Drospirenone-d4;2376035-94-6;Drospirenone-d4-1
|
| PubChem CID |
68873
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
552.2±50.0 °C at 760 mmHg
|
| 熔点 |
196-200ºC
|
| 闪点 |
241.6±30.2 °C
|
| 蒸汽压 |
0.0±1.5 mmHg at 25°C
|
| 折射率 |
1.610
|
| LogP |
3.15
|
| tPSA |
43.37
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
0
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
828
|
| 定义原子立体中心数目 |
10
|
| SMILES |
O1C(C([H])([H])C([H])([H])[C@@]21[C@@]1(C([H])([H])[H])C([H])([H])C([H])([H])[C@]3([H])[C@@]4(C([H])([H])[H])C([H])([H])C([H])([H])C(C([H])=C4[C@]4([H])C([H])([H])[C@]4([H])[C@@]3([H])[C@]1([H])[C@]1([H])C([H])([H])[C@@]12[H])=O)=O
|
| InChi Key |
METQSPRSQINEEU-HXCATZOESA-N
|
| InChi Code |
InChI=1S/C24H30O3/c1-22-6-3-12(25)9-17(22)13-10-14(13)20-16(22)4-7-23(2)21(20)15-11-18(15)24(23)8-5-19(26)27-24/h9,13-16,18,20-21H,3-8,10-11H2,1-2H3/t13-,14+,15-,16+,18+,20-,21+,22-,23+,24+/m1/s1
|
| 化学名 |
(1R,2R,4R,10R,11S,14S,15S,16S,18S,19S)-10,14-dimethylspiro[hexacyclo[9.8.0.02,4.05,10.014,19.016,18]nonadec-5-ene-15,5'-oxolane]-2',7-dione
|
| 别名 |
Drospirenona; Drospirenone; Dehydrospirorenone; dihydrospirorenone; drospirenone; Drospirenonum; ZK 3059; ZK30595
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 50~73 mg/mL (136.4~199.2 mM)
Ethanol: ~12 mg/mL (~32.7 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.82 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.82 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.82 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7286 mL | 13.6429 mL | 27.2859 mL | |
| 5 mM | 0.5457 mL | 2.7286 mL | 5.4572 mL | |
| 10 mM | 0.2729 mL | 1.3643 mL | 2.7286 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06039826 | Recruiting | Drug: Drospirenone Drug: Ethinyl Estradiol |
Overweight | Eli Lilly and Company | September 12, 2023 | Phase 1 |
| NCT04792385 | Active Recruiting |
Drug: E4/DRSP 15/3 mg combined tablet |
Safety | Estetra | December 28, 2020 | Phase 3 |
| NCT05675644 | Not yet recruiting | Drug: Drospirenone-only pill | Contraception | University of Colorado, Denver | February 2023 | Phase 2 |
| NCT05461573 | Recruiting | Drug: Drospirenone | Contraception Change in Bone Mineral Density |
Insud Pharma | August 2, 2022 | Phase 3 |
| NCT05156879 | Recruiting | Drug: Drospirenone ethinyl estradiol Drug: Aspirin |
Pelvic Pain | Women's Hospital School Of Medicine Zhejiang University |
December 23, 2021 | Phase 4 |