| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
VDCC/voltage-dependent calcium channel; HIV-1; COVID-19
|
|---|---|
| 体外研究 (In Vitro) |
在感染了 COVID-19 病毒的 Vero 细胞中,Ebselen(SPI-1005;0.4-100 μM;20-24 小时)在 10 μM 处理浓度下表现出有效的抗病毒作用。 Ebsele 将自身共价附着在 COVID-19 病毒 Mpro 催化二联体的 C145 位上[3]。依布硒啉通过减少进入的衣壳脱壳过程来抑制 HIV-1 生命周期的病毒进入后事件[4]。在小鼠大脑中,内源性肌醇单磷酸酶被依布硒啉抑制,依布硒啉也能穿透血脑屏障。依布硒啉 (ebselen) 抑制肌醇单磷酸酶 (IMPase) [5]。 Ebselen 抑制胰腺和肾癌细胞系的侵袭并抑制 QSOX1 的酶活性[6]。
|
| 体内研究 (In Vivo) |
依布硒啉(5、10 mg/kg;IP)以剂量依赖性方式减少 5-HT2 激动剂引起的头部抽搐[5]。
Ebselen在大脑中具有药理学活性[5] 为了确定依布硒是否可以穿过血脑屏障,从而在小鼠大脑中具有药理学活性,正如大鼠23所报道的那样,我们利用了依布硒在基于脑匀浆中IMPase活性的离体方法中的不可逆抑制特性(图2a)。由于最初将依布硒定为抑制剂的实验使用了重组人IMPase(图1b),我们首先需要确保重组小鼠IMPase具有酶活性。重组小鼠IMPase被锂、L-690、330和依必硒抑制(图2b)。在验证了依必硒抑制小鼠形式的IMPase后,我们证明在小鼠脑匀浆中,IMPase活性可被锂、L-690330和依必硒检测到并抑制(图2c)。在离体实验中,在腹腔注射依布硒后不同时间制备的脑匀浆中测量了IMPase活性(图2a)24。随着时间的推移,IMPase抑制作用逐渐发展,然后恢复到对照水平(图2d,e)。因此,全身给药依布硒可抑制整个动物小鼠脑中的IMPase。 Ebselen改变中枢神经系统的功能[5] Ebselen以剂量依赖的方式减少了5-HT2激动剂诱导的头部抽搐(图3a),这与前额叶皮层(图3b)和扣带皮层(图3c)中Arc mRNA(神经活动的分子标志物26)的表达减少有关。因此,依布硒能减弱与磷酸肌醇转换相关的皮质介导的5-HT2受体反应,正如IMPase抑制剂所预测的那样。 Ebselen对行为表现出锂样效应[5] 在开放式田间试验中(图3d),依布硒随时间减少饲养,然后恢复到基线水平(图3e),这一时间过程与体外试验中IMPase抑制的时间过程(图2e)以及口服给药后人体血浆依布硒浓度相似34。养育是一种探索性行为,与冲动有关33,冲动又与自杀念头和行为有关35。狂躁症也被苯丙胺诱导的多动所模拟(图3f)33,36。与锂37类似,依布硒以一种取决于苯丙胺剂量和依布硒剂量的方式减少了苯丙胺诱导的多动(图3g),锂37也是如此。基线活动度没有显著降低(单侧配对t检验:苯丙胺2 mg/kg和依布硒5 mg/kg,p=0.24;苯丙胺4 mg/kg和依布硒5 mg/kg;p=0.08) |
| 酶活实验 |
IMPase活性[5]
使用孔雀石绿测定法检测Ins1P水解的磷酸盐。对于体外检测,将重组HsIMPase(10 ng/孔)或MmIMPase(30 ng/孔)与Ins1P(1mM)在20μL Tris缓冲液(50 mM Tris-HCl、1 mM EGTA、3 mM MgCl2、150 mM KCl、0.5 mg/mL BSA和0.01%v/v Triton X pH 7.4)中孵育(1小时,37°C)。在595nm处测量样品和磷酸盐标准品的吸光度。对于离体试验,在有或没有LiCl(30 mM)的情况下,将脑匀浆(0.5 mg/mL)与Ins1P(0.1-2.4 mM)一起孵育(37°C,1小时),以确定IMPase的特异性活性。 化学文库与筛选[5] 在三种浓度的Ins1P下筛选化合物(100μM)。通过跨越六个数量级的浓度-抑制曲线证实了最初的打击。后续实验使用ebselen。对于化合物筛选,将100μM(在0.2%v/v DMSO中)的化合物与缓冲液中的IMPase一起孵育(10分钟,室温),然后加入Ins1P(1 mM)至最终体积为20μL,并进一步孵育(37°C,1小时)。磷酸酶浓度通过孔雀石绿测定法测定。LiCl和L-690330(Tocris)用作阳性对照。 rQSOX1活性测定[6] rQSOX1的巯基氧化酶活性通过DTT和RNAse A底物以及荧光过氧化氢指示剂高香草酸(HVA)得到证实[8]。在该试验中,在25°C、pH 7.5的PBS中,将150 nM rQSOX1添加到600μM硫醇中,这些硫醇来自还原的DTT或RNA酶A、1 mM HVA、1.4μM HRP和300μM EDTA。在最终反应体积为150μl的黑色96孔板上进行测定。使用FlexStation分光光度计在λex 320 nm和λem 420 nm下测量荧光信号10分钟)。在添加rQSOX1后,每隔20秒读取一次读数Ebselen在加入rQSOX1之前至少10分钟以250 nM-4μM的浓度加入反应中。 |
| 细胞实验 |
RT-PCR[3]
细胞类型: COVID-19 病毒感染的 Vero 细胞 测试浓度: 0.4、1.2、3.7、11.1、33.3、100 μM 孵育持续时间:20-24 小时 实验结果:在 10 μM 处理浓度下表现出强大的抗病毒作用。 依布硒治疗肿瘤细胞的生长动力学[6] 将1×104个细胞/孔的MIAPaCa-2、BXPC3、786-O和UOK1117在24孔板上一式两份。在加入新鲜培养基(未经处理)、载体(0.15%DMSO)或5μM–15μMebselen之前,将细胞粘附过夜。使用血细胞计数器和台盼蓝排斥法对细胞进行计数,以评估存活率。在第3天和第5天对细胞进行计数,并保存“漂浮物”(脱落和死亡的细胞)以确定整体存活率。在第5天时间点的第3天更换了介质;保存漂浮物并将其添加回每个井中,以便在第5天进行计数。存活率被确定为[1-(#死细胞/(#活细胞+#死细胞))*100]。误差表示为平均值的标准误差。与载体处理的细胞相比,使用配对T检验确定每个时间点的显著性。 跨井侵入试验[6] 在无血清培养基中,将2×104个MIAPaCa-2、BXPC3、786-O或UOK117细胞接种在复水的24孔侵袭试验插入物中,该插入物含有8μm的孔,上面覆盖有Matrigel;在加入ebselen或Vehicle之前,细胞粘附1小时。插入物在37°C下在含有完整培养基的孔中孵育20小时。用棉签去除非侵袭细胞,用100%甲醇固定膜,并用DAPI将其安装在载玻片上。通过手动计数DAPI染色的细胞核来确定侵袭细胞的总数。 |
| 动物实验 |
Animal/Disease Models: 20-25 g 10-12 week old male C57Bl6 mice[5]
Doses: 5, 10 mg/kg Route of Administration: IP Experimental Results: diminished 5-HT2 agonist-induced head twitches in a dose-dependent manner. Ex Vivo Mouse Brain Homogenate [5] Mice were injected with ebselen (10 mg/kg) or vehicle (4% w/v hydroxypropyl ß-cyclodextrin) and left for varying amounts of time before euthanization by cervical dislocation, or by CO2 followed by cervical dislocation. Brains were removed and frozen on dry ice immediately. One hemisphere was homogenized using a Precellys 24 bead mill homogenizer and diluted in Tris buffer (50 mM Tris HCl, 3 mM MgCl2, 150 mM KCl, 1 mM EGTA, 0.01% v/v Triton X pH 7.4) to a final concentration of 0.5 mg/mL. Ex vivo Inositol Measurement by Nuclear Magnetic Resonance [5] Mice were euthanized by cervical dislocation 1 h after administration of ebselen (10 mg/kg) or vehicle (4% w/v hydroxypropyl ß-cyclodextrin), then brains were extracted and frozen immediately on dry ice. Brains were weighed then homogenized using a Precellys 24 bead mill homogenizer. Acetonitrile was added to homogenate (1:1 v/v) to precipitate protein, the sample was centrifuged (13,000×g, 10 min), and the supernatant was prepared for NMR by lyophilization and reconstitution in D2O with 0.008% w/v 3- (trimethylsilyl)propionic 2233d acid sodium salt (600 mg/mL). Amphetamine-induced Hyperactivity [5] Mice were treated with ebselen or vehicle and immediately placed in Linton AM1053 X, Y, Z IR Activity Monitor for 1 h to habituate. Mice were then injected with d-amphetamine/saline and returned to the cage, and activity was monitored for an additional 1 h. Rearing behavior [5] Mice were injected with ebselen (10 mg/kg) or vehicle (4% w/v hydroxypropyl ß-cyclodextrin) and left for varying amounts of time before being placed in the Linton AM1053 X, Y, Z IR Activity Monitor for 30 mins while their activity was monitored. Rearing was measured by counting the number of beam breaks in upper grid. DOI-induced Head Twitches [5] Mice were placed in an arena and left to acclimatize to the novel environment. After 1 h, they were injected with vehicle or ebselen (5 or 10 mg/kg) followed 1 h later by the non-selective 5HT2A agonist 1- (2,5-dimethoxy-4 iodophenyl)-2-aminopropane (DOI, 2 mg/kg). Head twitches were recorded 5 min after agonist injection for 15 min. Mice were constantly monitored by a video camera, and behavioural recordings were analysed offline independently by two observers who were blind to the treatment. Nude mouse-human tumor xenograft model [6] For ebselen treatment of nude mice, three groups were tested: 1) 20% DMSO (vehicle), 2) 160 μg/day ebselen, and 3) 640 μg/day ebselen. 160 and 640 μg ebselen represent an equivalent dose of 150mg and 600mg for a 70 kg human, respectively. 1 × 106 MIAPaCa-2 cells were injected subcutaneously into each mouse as before, and tumors were allowed to grow for 3 days. Ebselen was then administered once daily through oral gavage for 28 days. Real-time tumor volume was determined through caliper measurement of tumors over the course of the study. |
| 毒性/毒理 (Toxicokinetics/TK) |
rat LD oral >4600 mg/kg European Patent Application., #0044971
mouse LDLo oral 5 gm/kg SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE; BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD; BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) Yakuri to Chiryo. Pharmacology and Therapeutics., 25(Suppl pig LD oral >2 gm/kg Yakuri to Chiryo. Pharmacology and Therapeutics., 25(Suppl |
| 参考文献 | |
| 其他信息 |
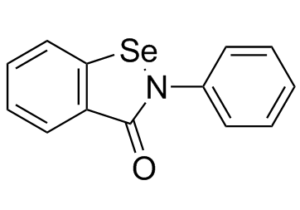
Ebselen is a benzoselenazole that is 1,2-benzoselenazol-3-one carrying an additional phenyl substituent at position 2. Acts as a mimic of glutathione peroxidase. It has a role as a neuroprotective agent, an apoptosis inducer, an anti-inflammatory drug, an antioxidant, a hepatoprotective agent, a genotoxin, a radical scavenger, an enzyme mimic, an EC 1.3.1.8 [acyl-CoA dehydrogenase (NADP(+))] inhibitor, an EC 1.8.1.12 (trypanothione-disulfide reductase) inhibitor, an EC 1.13.11.33 (arachidonate 15-lipoxygenase) inhibitor, an EC 1.13.11.34 (arachidonate 5-lipoxygenase) inhibitor, an EC 2.5.1.7 (UDP-N-acetylglucosamine 1-carboxyvinyltransferase) inhibitor, an EC 2.7.10.1 (receptor protein-tyrosine kinase) inhibitor, an EC 3.5.4.1 (cytosine deaminase) inhibitor, an EC 5.1.3.2 (UDP-glucose 4-epimerase) inhibitor, a ferroptosis inhibitor, an antifungal agent, an EC 3.4.22.69 (SARS coronavirus main proteinase) inhibitor, an anticoronaviral agent, an antibacterial agent, an antineoplastic agent and an EC 3.1.3.25 (inositol-phosphate phosphatase) inhibitor.
Ebselen has been investigated for the treatment and basic science of Meniere's Disease, Type 2 Diabetes Mellitus, and Type 1 Diabetes Mellitus. Ebselen is a organoselenium compound with anti-inflammatory, anti-oxidant and cytoprotective activity. Ebselen acts as a glutathione peroxidase mimetic and is thereby able to prevent cellular damage induced by reactive oxygen species (ROS). In addition, this agent inhibits the activity of a variety of enzymes including nitric oxide synthase (NOS), 5-lipoxygenase, cyclooxygenase, protein kinase C (PKC), NADPH oxidase and gastric H+/K+-ATPase. Furthermore, ebselen may be neuroprotective due to its ability to neutralize free radicals upon NMDA receptor activation thus, reducing lipoperoxidation mediated by glutamate-induced excitotoxicity. The selenoorganic compound ebselen, 2-phenyl-1,2-benzisoselenazol-3(2H)-one, exhibits activity as an enzyme mimic. The reaction catalyzed is that of a glutathione (GSH) peroxidase (i.e., the reduction of a hydroperoxide at the expense of thiol). The specificity for substrates ranges from hydrogen peroxide and smaller organic hydroperoxides to membrane-bound phospholipid and cholesterol hydroperoxides. In addition to glutathione, the thiol reductant cosubstrate can be dithioerythritol, N-acetylcysteine or dihydrolipoate, or other suitable thiol compounds. Ebselen also has properties such as free radical and singlet oxygen quenching. Model experiments in vitro with liposomes, microsomes, isolated cells, and organs show that the protection against oxidative challenge afforded by ebselen can be explained largely by the activity as GSH peroxidase mimic. Whether this also explains the known preliminary results in clinical settings is yet open. The metabolism and disposition of ebselen is presented in this review. The main point is that the selenium is not bioavailable, explaining the extremely low toxicity observed in animal studies. The occurrence of natural GPx mimics, ovothiol and related compounds, is briefly mentioned. [2] A new coronavirus, known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is the aetiological agent responsible for the 2019-2020 viral pneumonia outbreak of coronavirus disease 2019 (COVID-19)1-4. Currently, there are no targeted therapeutic agents for the treatment of this disease, and effective treatment options remain very limited. Here we describe the results of a programme that aimed to rapidly discover lead compounds for clinical use, by combining structure-assisted drug design, virtual drug screening and high-throughput screening. This programme focused on identifying drug leads that target main protease (Mpro) of SARS-CoV-2: Mpro is a key enzyme of coronaviruses and has a pivotal role in mediating viral replication and transcription, making it an attractive drug target for SARS-CoV-25,6. We identified a mechanism-based inhibitor (N3) by computer-aided drug design, and then determined the crystal structure of Mpro of SARS-CoV-2 in complex with this compound. Through a combination of structure-based virtual and high-throughput screening, we assayed more than 10,000 compounds-including approved drugs, drug candidates in clinical trials and other pharmacologically active compounds-as inhibitors of Mpro. Six of these compounds inhibited Mpro, showing half-maximal inhibitory concentration values that ranged from 0.67 to 21.4 μM. One of these compounds (ebselen) also exhibited promising antiviral activity in cell-based assays. Our results demonstrate the efficacy of our screening strategy, which can lead to the rapid discovery of drug leads with clinical potential in response to new infectious diseases for which no specific drugs or vaccines are available. [3] The human immunodeficiency virus type 1 (HIV-1) capsid plays crucial roles in HIV-1 replication and thus represents an excellent drug target. We developed a high-throughput screening method based on a time-resolved fluorescence resonance energy transfer (HTS-TR-FRET) assay, using the C-terminal domain (CTD) of HIV-1 capsid to identify inhibitors of capsid dimerization. This assay was used to screen a library of pharmacologically active compounds, composed of 1,280in vivo-active drugs, and identified ebselen [2-phenyl-1,2-benzisoselenazol-3(2H)-one], an organoselenium compound, as an inhibitor of HIV-1 capsid CTD dimerization. Nuclear magnetic resonance (NMR) spectroscopic analysis confirmed the direct interaction of ebselen with the HIV-1 capsid CTD and dimer dissociation when ebselen is in 2-fold molar excess. Electrospray ionization mass spectrometry revealed that ebselen covalently binds the HIV-1 capsid CTD, likely via a selenylsulfide linkage with Cys198 and Cys218. This compound presents anti-HIV activity in single and multiple rounds of infection in permissive cell lines as well as in primary peripheral blood mononuclear cells. Ebselen inhibits early viral postentry events of the HIV-1 life cycle by impairing the incoming capsid uncoating process. This compound also blocks infection of other retroviruses, such as Moloney murine leukemia virus and simian immunodeficiency virus, but displays no inhibitory activity against hepatitis C and influenza viruses. This study reports the use of TR-FRET screening to successfully identify a novel capsid inhibitor, ebselen, validating HIV-1 capsid as a promising target for drug development. [4] Lithium is the most effective mood stabilizer for the treatment of bipolar disorder, but it is toxic at only twice the therapeutic dosage and has many undesirable side effects. It is likely that a small molecule could be found with lithium-like efficacy but without toxicity through target-based drug discovery; however, therapeutic target of lithium remains equivocal. Inositol monophosphatase is a possible target but no bioavailable inhibitors exist. Here we report that the antioxidant ebselen inhibits inositol monophosphatase and induces lithium-like effects on mouse behaviour, which are reversed with inositol, consistent with a mechanism involving inhibition of inositol recycling. Ebselen is part of the National Institutes of Health Clinical Collection, a chemical library of bioavailable drugs considered clinically safe but without proven use. Therefore, ebselen represents a lithium mimetic with the potential both to validate inositol monophosphatase inhibition as a treatment for bipolar disorder and to serve as a treatment itself. [5] Quiescin sulfhydryl oxidase 1 (QSOX1) is a highly conserved disulfide bond-generating enzyme that is overexpressed in diverse tumor types. Its enzymatic activity promotes the growth and invasion of tumor cells and alters extracellular matrix composition. In a nude mouse-human tumor xenograft model, tumors containing shRNA for QSOX1 grew significantly more slowly than controls, suggesting that QSOX1 supports a proliferative phenotype in vivo. High throughput screening experiments identified ebselen as an in vitro inhibitor of QSOX1 enzymatic activity. Ebselen treatment of pancreatic and renal cancer cell lines stalled tumor growth and inhibited invasion through Matrigel in vitro. Daily oral treatment with ebselen resulted in a 58% reduction in tumor growth in mice bearing human pancreatic tumor xenografts compared to controls. Mass spectrometric analysis of ebselen-treated QSOX1 mechanistically revealed that C165 and C237 of QSOX1 covalently bound to ebselen. This report details the anti-neoplastic properties of ebselen in pancreatic and renal cancer cell lines. The results here offer a "proof-of-principle" that enzymatic inhibition of QSOX1 may have clinical relevancy. [6] |
| 分子式 |
C₁₃H₉NOSE
|
|
|---|---|---|
| 分子量 |
274.18
|
|
| 精确质量 |
274.984
|
|
| 元素分析 |
C, 56.95; H, 3.31; N, 5.11; O, 5.84; Se, 28.80
|
|
| CAS号 |
60940-34-3
|
|
| 相关CAS号 |
|
|
| PubChem CID |
3194
|
|
| 外观&性状 |
Light yellow to yellow solid powder
|
|
| 沸点 |
402.8±28.0 °C at 760 mmHg
|
|
| 熔点 |
178-181 °C
|
|
| 闪点 |
197.4±24.0 °C
|
|
| 蒸汽压 |
0.0±0.9 mmHg at 25°C
|
|
| LogP |
2.047
|
|
| tPSA |
22
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
1
|
|
| 可旋转键数目(RBC) |
1
|
|
| 重原子数目 |
16
|
|
| 分子复杂度/Complexity |
275
|
|
| 定义原子立体中心数目 |
0
|
|
| InChi Key |
DYEFUKCXAQOFHX-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C13H9NOSe/c15-13-11-8-4-5-9-12(11)16-14(13)10-6-2-1-3-7-10/h1-9H
|
|
| 化学名 |
2-phenyl-1,2-benzoselenazol-3-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.12 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.12 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.6472 mL | 18.2362 mL | 36.4724 mL | |
| 5 mM | 0.7294 mL | 3.6472 mL | 7.2945 mL | |
| 10 mM | 0.3647 mL | 1.8236 mL | 3.6472 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
 Ebselen induces local structural changes in CA-CTD.Antimicrob Agents Chemother.2016 Mar 25;60(4):2195-208. |
|---|
 Residues affected by ebselen in15N–labeled CA-CTD.Antimicrob Agents Chemother.2016 Mar 25;60(4):2195-208. |
 Mechanism of action of Ebselen.Antimicrob Agents Chemother.2016 Mar 25;60(4):2195-208. |