| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
PPAR-α (IC50 = 45 nM); PPAR-δ (IC50 = 175 nM)
PPAR-α (specific IC₅₀/Ki/EC₅₀ values ) [1][2][3][4] PPAR-δ (specific IC₅₀/Ki/EC₅₀ values ) [2][3][4] |
|---|---|
| 体外研究 (In Vitro) |
体外活性:Elafibranor(也称为 GFT505)正在开发作为 PPAR-α/PPAR-δ 双重激动剂,用于治疗 T2DM 和非酒精性脂肪肝疾病。 GFT505 具有活性代谢物 GFT1007,两者均对 PPAR-α 具有有效的激动剂活性,对 PPAR-δ 激酶测定具有较小程度的激动剂活性:Elafibranor 是一种 PPARα/δ 激动剂,EC50 分别为 45 和 175 nM。 GFT505 正在开发作为 PPAR-α/PPAR-δ 双重激动剂,用于治疗 T2DM 和非酒精性脂肪肝。 GFT505 具有活性代谢物 GFT1007,两者均对 PPAR-α 具有有效的激动剂活性,对 PPAR-δ 具有较小程度的激动剂活性。
Elafibranor(GFT505)在人皮肤干细胞衍生的NASH模型中可抑制脂肪生成和炎症反应;通过NFκB介导的通路,减少细胞内脂质积累,调节NASH特异性基因表达,降低caspase-3/7活性,并减少炎症标志物(CCL2、CCL5、CCL7、CCL8、CXCL5、CXCL8、IL1a、IL6、IL11)的表达和分泌[4] 在与代谢疾病相关的体外系统中,Elafibranor可改善胰岛素敏感性、葡萄糖稳态和脂质代谢,并减轻炎症[2][3] 转录组学分析证实,Elafibranor处理的体外NASH模型与人类NASH患者之间存在共同的调控基因和重叠的基因类别,验证了其人类相关性[4] |
| 体内研究 (In Vivo) |
Elafibranor(也称为 GFT505)是一种 PPARα/δ 双重激动剂,已在非酒精性脂肪性肝病 (NAFLD)/NASH 和肝纤维化疾病模型中显示出疗效。在大鼠中,GFT505 集中在肝脏中,肝外暴露有限,并经历了广泛的肠肝循环。 Elafibranor 通过作用于 NASH 发病机制中的多个途径来保护肝脏,减少脂肪变性、炎症和纤维化。 GFT505 改善肝功能障碍标志物,减少肝脏脂质积累,并抑制促炎症(白细胞介素 1 β、肿瘤坏死因子 α 和 F4/80)和促纤维化(转化生长因子 β、金属蛋白酶 2 组织抑制剂、I 型胶原、α 1)和 I 型胶原蛋白、α 2) 基因表达。
在意向治疗分析中,在方案定义的主要结局中,elafbranor组和安慰剂组之间没有显著差异。然而,与安慰剂组相比,120mg埃拉布诺组患者中NASH缓解而无纤维化恶化的比例更高(19% vs 12%;优势比= 2.31;95%置信区间:1.02-5.24;P = 0.045),基于修改后定义的事后分析。在非酒精性脂肪性肝病活动度评分≥4的患者(n = 234)的事后分析中,根据方案定义,依非布兰120mg缓解NASH的患者比例高于安慰剂(20% vs 11%;优势比= 3.16;95%置信区间:1.22-8.13;P = 0.018)和修改后的定义(19% vs 9%;优势比= 3.52;95%置信区间:1.32-9.40;P = .013)。接受艾非布诺120mg治疗后,NASH缓解的患者与NASH未缓解的患者相比,肝纤维化分期减少(主要终点应答者平均减少0.65±0.61,无应答者平均减少0.10±0.98;P < 0.001)。与安慰剂组相比,elafbranor 120 mg组的肝酶、血脂、葡萄糖谱和全身炎症标志物显著降低。Elafibranor耐受性良好,没有引起体重增加或心脏事件,但确实产生了轻度的、可逆的血清肌酐升高(与安慰剂相比,效应大小:增加4.31±1.19 μmol/L;P < 0.001)。 结论:对NASH患者试验数据的事后分析显示,根据修改的定义,在意向治疗分析和中度或重度NASH患者中,依非布诺(120mg /d,持续1年)可缓解NASH,无纤维化恶化。然而,在治疗人群的意图中,没有达到预定的终点。elafbranor耐受性良好,改善了患者的心脏代谢风险概况。ClinicalTrials.gov编号:NCT01694849.[2] Elafibranor在体外减弱了NASH的关键特征,并显著降低了脂质负荷以及炎症趋化因子的表达和分泌,炎症趋化因子在体内负责免疫细胞的募集。这种炎症反应的减少是由nfκ b介导的。总之,这种与人类相关的体外系统被证明是研究新型抗nash化合物的敏感测试工具。[4] 在db/db小鼠(糖尿病模型)中,Elafibranor经长达8周治疗后,可降低空腹血糖和糖化血红蛋白(HbA1c),改善胰岛素敏感性,并减少肝脏糖异生(与糖异生基因表达降低相关);其降糖效果与PPARγ激动剂罗格列酮和双重PPARα/γ激动剂阿格列扎相当[3] 在食蟹猴中,Elafibranor给药12个月未引起超声心动图和组织学上的心脏异常,也未导致血液学参数或骨髓分类细胞计数发生变化[3] 在一项针对无肝硬化NASH患者的国际随机双盲安慰剂对照II期临床试验(NCT01694849)中: - 120 mg/d Elafibranor治疗52周后,意向治疗人群中19%的患者实现NASH缓解且纤维化无恶化(安慰剂组为12%,修正定义,P=0.045)[2] - 在NAFLD活动评分≥4的患者中(n=234),120 mg/d Elafibranor按试验方案定义和修正定义实现NASH缓解的比例分别为20%(P=0.018)和19%(P=0.013),安慰剂组分别为11%和9%[2] - 经120 mg/d Elafibranor治疗后NASH缓解的患者,肝脏纤维化分期平均降低0.65±0.61(非缓解者平均增加0.10±0.98,P<0.001)[2] - 与安慰剂相比,120 mg/d Elafibranor可显著降低肝脏酶、脂质、葡萄糖谱和全身炎症标志物水平[2] |
| 酶活实验 |
Elafibranor 是 PPARα/δ 的激动剂,EC50 值依次为 45 和 175 nM。作为 PPAR-α/PPAR-δ 双重激动剂,GFT505 正在开发用于治疗非酒精性脂肪肝和 2 型糖尿病。 GFT505 的活性代谢物 GFT1007 对 PPAR-α 具有很强的激动剂活性,对 PPAR-δ 具有较小程度的激动剂活性。
|
| 细胞实验 |
hSKP-HPC细胞培养及脂肪变性和NASH体外模型的建立[4]
hSKP是在获得父母的知情同意和布鲁塞尔大学医学伦理委员会的批准后,从1至10岁的小男孩包皮环切术样本中分离出来的。这些细胞被培养并分化为肝细胞样细胞(hSKP-HPC),如先前文献所述。 通过将hSKP-HPC(24 h)暴露于胰岛素(100 nM)和葡萄糖(4.5 mg/mL),体外模拟脂肪变性。同时暴露于油酸钠(65 μM)和棕榈酸(45 μM)(24 h),并联合促炎(50 ng/mL肿瘤坏死因子(TNF)- α + 25 ng/mL白细胞介素(IL)-1β)[19]和促纤维化(8 ng/mL转化生长因子(TGF)-β1)细胞因子混合物,形成NASH条件。油酸钠与7% (w/v)无牛血清白蛋白(BSA)脂肪酸络合。用二甲亚砜(DMSO)溶解棕榈酸和elafibranor 。细胞同时暴露于NASH触发器和elafibranor (10 μM和30 μM)。所有样品中BSA和DMSO的最终浓度分别为0.14% (w/v)和0.15% (v/v)(除了油酸钠和棕榈酸浓度的测定(如图1A和B所示),其中分别使用1.4% (w/v) BSA和0.5% (v/v) DMSO)。 用于测定细胞因子和趋化因子的抗体阵列[4] 收集对照样本和暴露于NASH诱导剂和elafibranor (10 μM和30 μM)的细胞的新鲜细胞培养基,使用人细胞因子抗体阵列(120 Targets) 根据制造商的方案检测白细胞介素和趋化因子的分泌。使用Chemidoc™MP系统对杂交斑点进行可视化。采用Image Lab 5.0软件进行半定量数据分析(n = 5,除hSKP-HPC NASH +elafibranor 30 μM: n = 3)。 从人皮肤衍生前体细胞生成肝细胞,建立体外NASH模型;用脂肪生成因子(胰岛素、葡萄糖、脂肪酸)和促炎因子(IL-1β、TNF-α、TGF-β)处理细胞,诱导NASH样反应[4] 用Elafibranor处理体外NASH模型;通过评估细胞内脂质积累、NASH特异性基因表达(转录组学)、caspase-3/7活性以及炎症标志物(CCL2、CCL5等)的分泌,评价药物疗效[4] 进行NFκB通路分析,证实Elafibranor介导炎症反应减少的作用机制[4] |
| 动物实验 |
hApoE2 KI and hApoE2 KI/PPAR-α KO mice
30 mg/kg oral gavage Researchers report here the efficacy and safety of GFT505, a novel liver-targeted peroxisome proliferator-activated receptor alpha/delta (PPARα/δ) agonist, in the db/db mouse model of diabetes. Mice were treated with vehicle, GFT505, PPARγ agonist rosiglitazone or dual-PPARα/γ agonist aleglitazar for up to 8 weeks. All compounds comparably reduced fasting glycaemia and HbA1c and improved insulin sensitivity. The glucose-lowering effect of GFT505 was associated with decreased hepatic gluconeogenesis, correlating with reduced expression of gluconeogenic genes. In contrast with the PPARγ-activating drugs, treatment with GFT505 did not affect heart weight and did not increase plasma adiponectin concentrations. This absence of cardiac effects of GFT505 was confirmed after 12 months of administration in cynomolgus monkeys, by the absence of echocardiographic and histological findings. Moreover, long-term GFT505 administration to monkeys induced no change in haematological parameters or in bone marrow differential cell counts. Compared to PPARγ-activating drugs, the dual-PPARα/δ agonist GFT505 therefore shows an improved benefit/risk ratio, treating multiple features of type 2 diabetes without inducing the cardiac side-effects associated with PPARγ activation.[3] Patients with NASH without cirrhosis were randomly assigned to groups given elafibranor 80 mg (n = 93), elafibranor 120 mg (n = 91), or placebo (n = 92) each day for 52 weeks at sites in Europe and the United States. Clinical and laboratory evaluations were performed every 2 months during this 1-year period. Liver biopsies were then collected and patients were assessed 3 months later. The primary outcome was resolution of NASH without fibrosis worsening, using protocol-defined and modified definitions. Data from the groups given the different doses of elafibranor were compared with those from the placebo group using step-down logistic regression, adjusting for baseline nonalcoholic fatty liver disease activity score.[2] Treat db/db mice (diabetes model) with vehicle, Elafibranor, rosiglitazone, or aleglitazar for up to 8 weeks; monitor fasting glycaemia, HbA1c, insulin sensitivity, hepatic gluconeogenesis, and gluconeogenic gene expression [3] Administer Elafibranor to cynomolgus monkeys for 12 months; perform echocardiographic and histological examinations of the heart, and analyze haematological parameters and bone marrow differential cell counts [3] |
| 药代性质 (ADME/PK) |
Absorption
Following once-daily dosing, the steady state of elafibranor and its major active metabolite, GFT1007, was achieved within seven and fourteen days, respectively. The mean (SD) Cmax at steady state was 802 (443) ng/mL for elafibranor and 2058 (459) ng/mL for GFT1007. The mean (SD) AUC was 3758 (1749) ng x h/mL for elafibranor and 11985 (7149) ng x h/mL for GFT1007. Following once-daily dosing of 80 mg in patients with PBC, the median time to peak plasma concentrations (Tmax) of elafibranor and GFT1007 was 1.25 hours (range: 0.5-2 hours). When administered with a high-fat and high-calorie meal, Tmax was delayed by 30 minutes for elafibranor and by 1-hour for GFT1007 compared to in fasted conditions. Under fed condition, mean Cmax and AUC of elafibranor decreased by 50% and 15% respectively and mean Cmax of GFT1007 decreased by 30%, but the AUC was not affected compared to fasted conditions. The difference was not clinically meaningful. Route of Elimination Following a single 120 mg oral dose (1.5 times the recommended dose) of 14C-radiolabelled elafibranor in healthy subjects, approximately 77.1% of the dose was recovered in feces, primarily as elafibranor (56.7% of the administered dose) and its major metabolite GFT1007 (6.08% of the administered dose). Approximately 19.3% was recovered in urine, primarily as glucuronide conjugate GFT3351 (11.8% of the administered dose). A negligible amount of unchanged elafibranor or GFT1007 was detectable in the urine. Biliary excretion of elafibranor in humans was suggested by the excretion of 60% of orally administered elafibranor in the bile of rats. Volume of Distribution The mean apparent volume of distribution (Vd/F) of elafibranor in healthy subjects was 4731 L, following a single dose of 80 mg under fasted conditions. Clearance The mean apparent total clearance (CL/F) of elafibranor was 50.0 L/h after a single 80 mg dose under fasted conditions. Protein Binding Elafibranor and GFT1007 were approximately 99.7% bound to plasma proteins, mostly to serum albumin. Metabolism / Metabolites Elafibranor is extensively metabolized to form a major active metabolite, GFT1007, the chemical structure of which has not yet been characterized. The mean systemic exposure (AUC) to GFT1007 was 3.2-fold higher than that of elafibranor at steady state. GFT3351, an acyl glucuronide conjugate, is a major inactive metabolite that consists of four stereoisomers. In vitro studies showed that elafibranor was metabolized by 15-ketoprostaglandin 13-Δ reductase (PTGR1), a cytosolic enzyme, to form GFT1007. Elafibranor was also metabolized by CYP2J2, UGT1A3, UGT1A4, and UGT2B7. GFT1007 was further metabolized by CYP2C8, UGT1A3, and UGT2B7. Biological Half-Life Following a single 80 mg dose under fasted conditions, median elimination half-life was 70.2 hours (range 37.1 to 92.2 hours) for elafibranor, and 15.4 hours (range 9.39 to 21.7 hours) for major active metabolite GFT1007. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In preregistration clinical trials, elafibranor was found to decrease both serum aminotransferase and alkaline phosphatase elevations in a high proportion of patients with PBC. In preliminary dose-finding studies in healthy volunteers, elevations of ALT and AST levels above 5 times the upper limit of normal (ULN) were found to be dose related and occurred in one-third of subjects exposed to doses above 120 mg daily. In contrast, in clinical trials of elafibranor in doses of 80 mg daily in patients with NASH and PBC, ALT elevations above 5 times ULN occurred in only 1% to 2% of patients, typically arising within the first few months of therapy and resolving spontaneously without drug interruption and without jaundice or symptoms. Careful assessment of cases with ALT elevations concluded that 3 were possibly due to drug induced injury, 2 among 138 patients with PBC and 1 among 1433 patients with NASH. Among patients with myalgia and CPK elevations during elafibranor therapy, one patient with preexisting cirrhosis who was also taking a statin, developed jaundice [5.5 mg/dL] with elevations in ALT [300 U/L] and AST [828 U/L] concurrent with rhabdomyolysis [CPK 12,647 U/L] and subsequently suffered hepatic decompensation. The incidence of gallstones and cholecystitis also may be increased with elafibranor therapy. Rare instances of drug induced liver injury are known to occur with other PPARα (fenofibrate, bezafibrate) and PPARγ (pioglitazone, rosiglitazone) agonists. Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury). Elafibranor is well-tolerated in Phase II clinical trial; it does not cause weight gain or cardiac events but induces a mild, reversible increase in serum creatinine (effect size vs placebo: 4.31 ± 1.19 μmol/L, P<0.001) [2] Unlike PPARγ-activating drugs (rosiglitazone, aleglitazar), Elafibranor does not affect heart weight or increase plasma adiponectin concentrations in db/db mice [3] Long-term (12 months) administration of Elafibranor to cynomolgus monkeys shows no cardiac toxicity, haematological abnormalities, or bone marrow toxicity [3] |
| 参考文献 |
|
| 其他信息 |
Elafibranor (code name GFT505) is a multimodal and pluripotent medication for treatment of atherogenic dyslipidemia for an overweight patient with or without diabetes. It is an oral treatment that acts on the 3 sub-types of PPAR (PPARa, PPARg, PPARd) with a preferential action on PPARa. As of February 2016, elafibranor has completed 8 clinical trials and a phase III is in progress.
Elafibranor is an orally available peroxisome proliferator-activated receptor agonist that is used in combination with ursodeoxycholic acid to treat primary biliary cholangitis. Elafibranor therapy is associated with rare instances of worsening of liver enzymes during therapy but has not been convincingly linked to episodes of clinically apparent liver injury with jaundice. Drug Indication Investigated for use/treatment in atherosclerosis and diabetes mellitus type 2. Treatment of primary biliary cholangitis Treatment of non-alcoholic fatty liver disease (NAFLD) including non-alcoholic steatohepatitis (NASH) Mechanism of Action GFT505 is an oral treatment that acts on the 3 sub-types of PPAR (PPARa, PPARg, PPARd) with a preferential action on PPARa. It has a sophisticated mechanism of action. It is able to differentially recruit cofactors to the nuclear receptor, which subsequently lead to differential regulation of genes and biological effect. Therefore, the ability to identify and profile the activity of selective nuclear receptor modulator (SNuRMs) is a powerful approach to select innovative drug candidates with improved efficacy and diminished side effects. These pluripotent and multimodal molecules have significant positive effects on obesity, insulin-resistance and diabetes, atherosclerosis, inflammation, and the lipid triad (increasing of HDL cholesterol, lowering of triglycerides and LDL cholesterol).\n \nIntroduction: The fibrates have been used for many years to treat dyslipidemias and have also recently been shown to have anti-inflammatory effects. They are relatively weak PPAR-α agonists and do have some adverse effects. Novel compounds are in development, which are selective PPAR modulators (SPPARMs) and have more potent PPAR-α agonist activity. These may prove to have advantages in the treatment of dyslipidemia, insulin resistance and non-alcoholic fatty liver disease (NAFLD).\n\nAreas covered: This review focuses on PPAR-α agonists or SPPARMs in development describing the preclinical and early clinical studies. The information was obtained by searching the published literature and abstracts from recent meetings. Ongoing clinical trials were identified using the Clinicaltrial.gov database.\n\nExpert opinion: There is still a need for new drugs to treat atherogenic dyslipidemia. The highly potent and selective PPAR-α agonist K-877 has shown beneficial effects on atherogenic dyslipidemia and absence of some adverse effects seen with fibrates. The dual PPAR-α/PPAR-δ agonist GFT-505 has shown favorable results in improving atherogenic dyslipidemia and insulin resistance and appears to be a potential candidate for the treatment of NAFLD. Long-term trials are needed to assess the safety and efficacy of these new agents for cardiovascular and liver outcomes.[1] \n\n\nNon-alcoholic steatohepatitis (NASH) is characterized by hepatocellular steatosis with concomitant hepatic inflammation. Despite its pandemic proportions, no anti-NASH drugs have been approved yet. This is partially because drug development is decelerated due to the lack of adequate tools to assess the efficacy of potential new drug candidates. The present study describes the development and application of a new preclinical model for NASH using hepatic cells generated from human skin-derived precursors. Exposure of these cells to lipogenic (insulin, glucose, fatty acids) and pro-inflammatory factors (IL-1β, TNF-α, TGF-β) resulted in a characteristic NASH response, as indicated by intracellular lipid accumulation, modulation of NASH-specific gene expression, increased caspase-3/7 activity and the expression and/or secretion of inflammatory markers, including CCL2, CCL5, CCL7, CCL8, CXCL5, CXCL8, IL1a, IL6 and IL11. The human relevance of the proposed NASH model was verified by transcriptomics analyses that revealed commonly modulated genes and the identification of the same gene classes between the in vitro system and patients suffering from NASH. The application potential of this in vitro model was demonstrated by testing elafibranor, a promising anti-NASH compound currently under clinical phase III trial evaluation. Elafibranor attenuated in vitro key features of NASH, and dramatically lowered lipid load as well as the expression and secretion of inflammatory chemokines, which in vivo are responsible for the recruitment of immune cells. This reduction in inflammatory response was NFκB-mediated. In summary, this human-relevant, in vitro system proved to be a sensitive testing tool for the investigation of novel anti-NASH compounds.[4] Elafibranor (GFT505) is a dual PPAR-α/δ agonist and liver-targeted investigational drug [1][3] It is being evaluated in clinical Phase III trials for the treatment of non-alcoholic steatohepatitis (NASH) [4] Elafibranor exerts anti-diabetic effects in type 2 diabetes models and improves atherogenic dyslipidemia and insulin resistance, making it a potential candidate for NAFLD/NASH treatment [1][3] Its mechanism of action involves modulation of lipid metabolism, glucose homeostasis, and inflammation, with anti-inflammatory effects mediated via NFκB pathway inhibition [2][3][4] In clinical trials, Elafibranor 120 mg/d shows better efficacy than 80 mg/d; the predefined primary outcome is not met in intention-to-treat population, but post-hoc analyses demonstrate significant benefits in specific patient subgroups [2] Pharmacodynamics Elafibranor inhibits bile acid synthesis. It was also shown to improve insulin sensitivity, glucose homeostasis, and lipid metabolism. In patients with PBC, elafibranor reduced the mean levels of alkaline phosphatase (ALP). An in vitro PPAR functional assay showed that both elafibranor and GFT1007 produced activation of PPARalpha (EC50 = 46 nM and 14 nM, respectively, and Emax = 56% and 61%, respectively, relative to reference agonists). The potency of elafibranor and GFT1007 for PPAR-alpha activation exceeded the respective potencies for PPAR-gamma and PPAR-delta activation by approximately 3- to 8-fold. Elafibranor is a dual peroxisome proliferator-activated receptor (PPAR) α and β/δ agonist that works to inhibit bile acid synthesis. On June 10, 2024, elafibranor was granted accelerated approval by the FDA for the treatment of primary biliary cholangitis (PBC). The drug was also approved by the EMA on September 23, 2024. Elafibranor is a Peroxisome Proliferator-activated Receptor Agonist. The mechanism of action of elafibranor is as a Peroxisome Proliferator-activated Receptor Agonist, and Cytochrome P450 3A4 Inducer. Elafibranor is an orally bioavailable agonist of peroxisome proliferator-activated receptor (PPAR)-alpha (PPARa) and -delta (PPARd), with bile acids reducing activity. Upon oral administration, elafibranor, and its main active metabolite GFT1007, target, bind to and activate PPARa and PPARd in the liver. This induces the expression of fibroblast growth factor 21 (FGF21) and downregulates CYP7A1, the key enzyme responsible for the synthesis of bile acids from cholesterol. By reducing CYP7A1 expression, bile acid synthesis is reduced. This leads to reduced bile toxicity, and reduced inflammation and scarring associated with primary biliary cholangitis (PBC). ELAFIBRANOR is a small molecule drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 2024 and is indicated for cholangitis and has 6 investigational indications. |
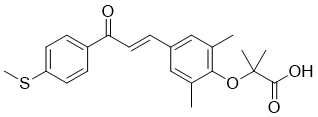
| 分子式 |
C22H24O4S
|
|---|---|
| 分子量 |
384.49
|
| 精确质量 |
384.139
|
| 元素分析 |
C, 68.73; H, 6.29; O, 16.64; S, 8.34
|
| CAS号 |
923978-27-2
|
| 相关CAS号 |
824932-88-9; 923978-27-2;
|
| PubChem CID |
9864881
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
569.0±50.0 °C at 760 mmHg
|
| 闪点 |
297.9±30.1 °C
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
| 折射率 |
1.606
|
| LogP |
5.63
|
| tPSA |
88.9
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
537
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O(C1C(C)=CC(/C=C/C(C2C=CC(SC)=CC=2)=O)=CC=1C)C(C)(C)C(=O)O
|
| InChi Key |
AFLFKFHDSCQHOL-IZZDOVSWSA-N
|
| InChi Code |
InChI=1S/C22H24O4S/c1-14-12-16(13-15(2)20(14)26-22(3,4)21(24)25)6-11-19(23)17-7-9-18(27-5)10-8-17/h6-13H,1-5H3,(H,24,25)/b11-6+
|
| 化学名 |
2-[2,6-dimethyl-4-[(E)-3-(4-methylsulfanylphenyl)-3-oxoprop-1-enyl]phenoxy]-2-methylpropanoic acid
|
| 别名 |
Elafibranor; 923978-27-2; 824932-88-9; Iqirvo; 2J3H5C81A5; 923978-27-2; GFT505; GFT-505; 824932-88-9; Elafibranor(GFT505); Elafibranor [INN]; Elafibranor [USAN]; GFT505; Elafibranor; GFT 505
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.87 mg/mL (7.46 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.17 mg/mL (5.64 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 21.7 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.17 mg/mL (5.64 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.17 mg/mL (5.64 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 21.7 mg/mL澄清DMSO储备液加入900 μL玉米油中,混合均匀。 配方 5 中的溶解度: ≥ 2.17 mg/mL (5.64 mM)(饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加),澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 21.7 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6008 mL | 13.0042 mL | 26.0085 mL | |
| 5 mM | 0.5202 mL | 2.6008 mL | 5.2017 mL | |
| 10 mM | 0.2601 mL | 1.3004 mL | 2.6008 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Study to Compare the Level of Elafibranor in Blood After Repeat Administration in Japanese and Non-Asian Healthy Participants
CTID: NCT05543369
Phase: Phase 1 Status: Completed
Date: 2023-07-18
Elafibranor-induced changes in glucose homeostasis markers in type 2 diabetic patients.Gastroenterology.2016 May;150(5):1147-1159.e5. |
|---|
Changes from baseline in liver enzymes (A−C) and plasma lipids (D−F) in treatment groups of the Per Protocol set.
Overall improvement in liver histology in patients who achieved the primary outcome according to the modified definition of response in the elafibranor 120-mg arm. |
Changes from baseline in inflammatory markers (Sup2A) and in noninvasive scores of fibrosis and steatosis (Sup2B) in treatment groups in the per protocol analysis (n = 237).Gastroenterology.2016 May;150(5):1147-1159.e5. |