| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
| 体外研究 (In Vitro) |
体外活性:Elagolix 是一种短效非肽 GnRH 拮抗剂,具有口服生物活性,与可注射的长效 GnRH 激动剂和拮抗剂不同,它对卵巢雌激素产生产生剂量依赖性抑制,即从较低剂量的部分抑制到完全抑制较高剂量时有抑制作用。激酶测定:Elagolix(以前称为 NBI56418 和 ABT-620;商品名:Orilissa)是一种有效的、特异性的、口服生物可利用的、非肽类促性腺激素释放激素受体 (GnRHR) 拮抗剂,KD 为 54 pM。 2018 年 7 月 23 日,Elagolix 被 FDA 批准用于治疗与子宫内膜异位症相关的中度至重度疼痛。 Elagolix 是一种短效 GnRH 拮抗剂,对卵巢雌激素产生产生剂量依赖性抑制,即从较低剂量的部分抑制到较高剂量的完全抑制。 Elagolix 因其非肽性质和口服生物利用度而被认为是新型 GnRH 抑制剂的领跑者,被称为第二代。细胞测定:
|
||
|---|---|---|---|
| 体内研究 (In Vivo) |
这项随机双盲研究采用 24 周治疗期和 24 周治疗后期,评估了恶拉戈利(每天 150 毫克,每天两次 75 毫克)与皮下长效醋酸甲羟孕酮 (DMPA-SC) 对骨矿物质密度的影响(BMD),患有子宫内膜异位症相关疼痛的女性 (n = 252)。所有治疗均在第 24 周引起 BMD 相对于基线的最小平均变化(恶拉戈利 150 mg:-0.11%/-0.47%,恶拉戈利 75 mg:-1.29%/-1.2%,DMPA-SC:0.99%/-1.29%)脊柱和全髋关节),在第 48 周(治疗后)有类似或更少的变化。通过综合盆腔体征和症状评分 (CPSSS) 和视觉模拟量表进行评估,恶拉戈利与子宫内膜异位症相关疼痛的改善相关,包括在 CPSSS 的痛经和非经期盆腔疼痛方面与 DMPA-SC 相比具有统计学上的非劣效性。恶拉戈利组最常见的不良事件 (AE) 是头痛、恶心和鼻咽炎,而 DMPA-SC 组最常见的 AE 是头痛、恶心、上呼吸道感染和情绪波动。这项研究表明,与 DMPA-SC 类似,恶拉戈利治疗在 24 周内对 BMD 的影响最小,并且对子宫内膜异位症相关疼痛具有相似的疗效。
|
||
| 酶活实验 |
Elagolix 是一种非肽、口服生物利用度强、选择性强、KD 为 54 pM 的药物,是促性腺激素释放激素受体 (GnRHR) 的拮抗剂。它以前被称为NBI56418和ABT-620;商品名:奥丽丽莎。 FDA 于 2018 年 7 月 23 日批准 Elagolix 用于治疗中度至重度子宫内膜异位症相关疼痛。 Elagolix 是一种短效 GnRH 拮抗剂,以剂量依赖性方式抑制卵巢雌激素的产生,这意味着较高剂量会导致完全抑制,而较低剂量只会导致部分抑制。 Elagolix 的非肽结构和口服生物利用度使其成为一类新型 GnRH 抑制剂(称为第二代抑制剂)的领导者。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The Tmax of elagolix is reported as being 1.0 hours. The effect of a high-fat meal (relative to fasting) can result in a reduction of the AUC and Cmax by as much as 24% and 36%, respectively. The primary route of elimination of elagolix is via hepatic metabolism. The apparent volume of distribution at steady state (Vdss/F) of elagolix is reported to be 1674 for a 150 mg daily regimen and 881 for a 200 mg twice daily regimen. The oral clearance (CL/F) of elagolix is 123 L/hr for a 150 mg once daily regimen and 144 L/hr for a 200 mg twice daily regimen. Metabolism / Metabolites Elagolix is predominantly metabolized by the CYP3A family of isoenzymes despite participating in minor metabolic pathways with the CYP2D6, CYP2C8, and uridine glucuronosyl transferases (UGTs) enzymes as well. The primary metabolite of elagolix, referred to as NBI-61962 (R-(+)-4-{2-[5-(2-fluoro-3-hydroxy-phenyl)-3-(2-fluoro-6-trifluoromethyl-benzyl)-4-methyl-2,6-dioxo-3,6-dihydro-2H-pyrimidin-1-yl]-1-phenyl-ethylamino}-butyrate), is not believed to possess any significant biologic activity due to its low plasma exposure and an observed potency that is exceptionally less than the parent elagolix compound (Ki value of 3.5 compared to 0.9 nM). Biological Half-Life The terminal phase elimination half-life of elagolix is recorded as being 4 to 6 hours. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Elagolix therapy has been associated with serum enzyme elevations in a small proportion of patients, rates of ALT elevations above 3 times the upper limit of normal being 0.2% with 150 mg once daily and 1.1% with 200 mg twice daily. The elevations, however, are generally mild and self-limited, resolving even without dose adjustment. Occasional patients require drug discontinuation because of serum enzyme elevations, but there were no instances of liver injury with jaundice or clinically apparent acute liver injury in the preregistration-controlled trials. Since its approval and more widescale use, there have been no published reports of clinically apparent liver injury attributed to elagolix. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of elagolix during breastfeeding. Elagolix is 80% protein bound, has a half-life of 4 to 6 hours, and it is a peptide that is likely digested in the infant's gastrointestinal tract, so it is unlikely to reach clinically important levels in infant serum. However, because no information is available on the use of elagolix during breastfeeding caution should be used, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding The percentage bound to human plasma proteins for elagolix has been documented as 80%. |
||
| 参考文献 | |||
| 其他信息 |
Pharmacodynamics
During a three menstrual cycle study in healthy women, an elagolix 150 mg once daily regimen and a 200 mg twice daily regimen resulted in an ovulation rate of about 50% and 32%, respectively. In Phase 3 trials in women with endometriosis, elagolix caused a dose-dependent reduction in median estradiol concentrations to approximately 42 pg/mL for the 150 mg once daily regimen and 12 pg/mL for the 200 mg twice daily regimen. Furthermore, the effect of elagolix on the QTc interval was investigated in a randomized, placebo- and positive-controlled, open-label, single-dose, crossover thorough QTc study in 48 healthy adult premenopausal women. Elagolix concentrations in subjects administered a single dose of 1200 mg was seventeen times higher than the concentration in subjects given elagolix 200 mg twice daily. Nevertheless, there was no clinically relevant prolongation of the QTc interval. |
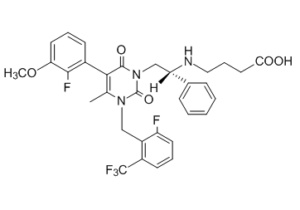
| 分子式 |
C32H30F5N3O5
|
|
|---|---|---|
| 分子量 |
631.59
|
|
| 精确质量 |
631.21
|
|
| 元素分析 |
C, 60.85; H, 4.79; F, 15.04; N, 6.65; O, 12.67
|
|
| CAS号 |
834153-87-6
|
|
| 相关CAS号 |
Elagolix sodium; 832720-36-2
|
|
| PubChem CID |
11250647
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
728.6±70.0 °C at 760 mmHg
|
|
| 闪点 |
394.5±35.7 °C
|
|
| 蒸汽压 |
0.0±2.5 mmHg at 25°C
|
|
| 折射率 |
1.567
|
|
| LogP |
7.2
|
|
| tPSA |
102.56
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
11
|
|
| 可旋转键数目(RBC) |
12
|
|
| 重原子数目 |
45
|
|
| 分子复杂度/Complexity |
1080
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
CC1N(CC2C(F)=CC=CC=2C(F)(F)F)C(=O)N(C[C@@H](C2C=CC=CC=2)NCCCC(=O)O)C(=O)C=1C1C=CC=C(OC)C=1F
|
|
| InChi Key |
EAUOKZIVMZVQL-VWLOTQADSA-N
|
|
| InChi Code |
InChI=1S/C32H30F5N3O5/c1-19-28(21-11-6-14-26(45-2)29(21)34)30(43)40(18-25(20-9-4-3-5-10-20)38-16-8-15-27(41)42)31(44)39(19)17-22-23(32(35,36)37)12-7-13-24(22)33/h3-7,9-14,25,38H,8,15-18H2,1-2H3,(H,41,42)/t25-/m0/s1
|
|
| 化学名 |
4-[[(1R)-2-[5-(2-fluoro-3-methoxyphenyl)-3-[[2-fluoro-6-(trifluoromethyl)phenyl]methyl]-4-methyl-2,6-dioxopyrimidin-1-yl]-1-phenylethyl]amino]butanoic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (3.96 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (3.96 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (3.96 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5833 mL | 7.9165 mL | 15.8331 mL | |
| 5 mM | 0.3167 mL | 1.5833 mL | 3.1666 mL | |
| 10 mM | 0.1583 mL | 0.7917 mL | 1.5833 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03271489 | Active Recruiting |
Other: Elagolix Placebo Drug: Elagolix |
Heavy Menstrual Bleeding Uterine Fibroids |
AbbVie | September 13, 2017 | Phase 3 |
| NCT04630990 | Active Recruiting |
N/A | Endometriosis | AbbVie | December 14, 2020 | N/A |
| NCT04856306 | Active Recruiting |
Drug: Elagolix Oral Product Other: Groups 1 and 2 myomectomy and uterine artery embolization, respectively, are surgical/procedure |
Heavy Menstrual Bleeding Fibroid Uterus |
Medstar Health Research Institute |
April 12, 2021 | N/A |
| NCT06076486 | Recruiting | Drug: Elagolix Drug: Elagolix placebo |
Endometriosis | Nanjing Chia-tai Tianqing Pharmaceutical |
September 14, 2023 | Phase 3 |
| NCT05648669 | Recruiting | Drug: Elagolix Drug: Elagolix placebo |
Endometriosis | Qilu Pharmaceutical (Hainan) Co., Ltd. |
September 4, 2022 | Phase 3 |