| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
Eliglustat tartrate 具有抑制靶酶的能力,且效力良好,IC50 为 24 nM [1]。在 72 小时内向 K562 或 B16/F10 细胞中添加 Genz-112638 (0.6-1000 nM) 会延迟抑制 GM1 和 GM3 细胞表面,平均 IC50 为 24 nM(范围 14-1000 nM) )在K562细胞中。 34 nM),B16/F10 细胞中 GM3 抑制的平均 IC50 值为 29 nM(范围 12-34 nM)。 48 纳米)[1]。
|
|---|---|
| 体内研究 (In Vivo) |
与年龄匹配的对照动物相比,治疗前底物大量积累的小鼠的阶梯神经酰胺水平较低,脾、肺和心脏中的戈谢细胞也较少[1]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Eliglustat administered in multiple doses of 84 mg twice daily had a Cmax of 12.1 to 25.0 ng/mL in CYP2D6 extensive metabolizers (EMs), 44.6 ng/mL in CYP2D6 intermediate metabolizers (IMs), and 113 to 137 ng/mL in CYP2D6 poor metabolizers (PMs). The median Tmax was 1.5-2 hr in CYP2D6 EMs, 2 hr in CYP2D6 IMs, and 3 hr in CYP2D6 PMs. The AUCtau was 76.3-143 ng∙hr/mL in CYP2D6 EMs, 306 ng∙hr/mL in CYP2D6 IMs, and 922-1057 ng∙hr/mL in CYP2D6 PMs. In CYP2D6 EMs, the pharmacokinetics of eliglustat is time-dependent, and for doses that range between 42 and 294 mg, exposure increases in a more than dose-proportional fashion. In CYP2D6 PMs, eliglustat pharmacokinetics is linear and time-independent. In a steady state, the systemic exposure of 84 mg eliglustat twice daily is 7- to 9-fold higher in CYP2D6 PMs compared to EMs. Following the oral administration of a single 84 mg dose of eliglustat, bioavailability in CYP2D6 EMs was lower than 5%. The low oral bioavailability of eliglustat suggests the role of transporters and/or an extensive first-pass metabolism. Eliglustat can be taken with or without food. In CYP2D6 EMs, severe renal impairment did not have an effect on eliglustat pharmacokinetics. The effect of renal impairment on eliglustat pharmacokinetics was not evaluated in CYP2D6 IMs, CYP2D6 PMs or CYP2D6 EMs with end-stage renal failure. Compared to CYP2D6 EMs with normal hepatic function, Cmax and AUC were 1.2-fold higher in CYP2D6 EMs with mild hepatic impairment, while Cmax and AUC were 2.8- and 5.2-fold higher, respectively, in CYP2D6 EMs with moderate hepatic impairment. The effect of mild and moderate hepatic impairment in CYP2D6 IMs and PMs, and the effect of severe hepatic impairment were not evaluated. Eliglustat is mainly excreted in urine (42%) and feces (51%) as metabolites after oral administration. In CYP2D6 extensive metabolizers (EM), the volume of distribution of eliglustat administered IV was 835 L. In healthy CYP2D6 extensive metabolizers (EMs) administered 42 mg of eliglustat IV (0.5 times the recommended oral dose), clearance was 88 L/h (80-105 L/h). Metabolism / Metabolites Eliglustat is mostly metabolized by CYP2D6, and to a lower extent, by CYP3A4. In patients that are CYP2D6 poor metabolizers (PMs), eliglustat is mainly metabolized by CYP3A4. The primary metabolic pathways of eliglustat involve the sequential oxidation of the octanoyl moiety and the 2,3-dihydro-1,4-benzodioxane moiety. The combination of these two pathways results in the production of several oxidative metabolites. After evaluating the potency of eliglustat metabolites, it was determined that none of them were active. Genz-399240 (M24) was identified as the major metabolite of eliglustat, while the rest of the metabolites contributed to less than 10% of total drug-related exposures. Genz-399240 (M24) did not show any major off-target effects; therefore, a transporter substrate specificity characterization was not performed. Biological Half-Life Eliglustat has a terminal elimination half-life of 6.5 hours in CYP2D6 extensive metabolizers (EMs) and 8.9 h in CYP2D6 poor metabolizers (PMs). |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In placebo controlled trials, liver test abnormalities were no more common with eliglustat than with placebo treatment, and what abnormalities occurred were mild and resolved spontaneously usually without need for dose interruption. During these premarketing clinical trials and since its more widespread clinical availability, no instances of acute liver injury with jaundice have been reported attributable to eliglustat. However, the total clinical experience with eliglustat use has been limited. Likelihood score: E (unlikely cause of clinically apparent liver injury, but experience with its use is limited). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because there is no published experience with eliglustat during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding In plasma, the protein binding of eliglustat goes from 76% to 83%. |
| 参考文献 | |
| 其他信息 |
Pharmacodynamics
Eliglustat is a specific inhibitor of glucosylceramide synthase (IC50 =10 ng/mL). In vitro studies suggest that eliglustat has minimal or no off-target activity against other glycosidases, such as α-glucosidase I and II, and lysosomal and non-lysosomal glucosylceramidases. At 8 times the recommended dose (800 mg) and a mean peak concentration of 237 ng/mL, eliglustat did not have a clinically significant effect on QTc prolongation. However, modelling of PK/PD data predicts that at a plasma concentration of 500 ng/mL, PR, QRS and QTcF intervals increase 22, 7, and 13 msec, respectively. Since high plasma concentrations of eliglustat may increase the risk of cardiac arrhythmias, there are warnings and precautions for patients taking CYP2D6 or CYP3A4 inhibitors, those with specific CYP2D6 metabolizer status and different degrees of hepatic impairment. Depending on each case, the use of this drug is contraindicated, to be avoided, or requires dosage adjustment. Patients with preexisting cardiac disease (congestive heart failure, recent acute myocardial infarction, bradycardia, heart block, ventricular arrhythmia), long QT syndrome, or those taking Class IA or Class II antiarrhythmic drugs are advised to avoid eliglustat. |
| 精确质量 |
404.267
|
|---|---|
| CAS号 |
491833-29-5
|
| 相关CAS号 |
Eliglustat hemitartrate;928659-70-5;Eliglustat-d15 tartrate;1884556-84-6;Eliglustat-d4
|
| PubChem CID |
23652731
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
615.5±55.0 °C at 760 mmHg
|
| 闪点 |
326.1±31.5 °C
|
| 蒸汽压 |
0.0±1.9 mmHg at 25°C
|
| 折射率 |
1.543
|
| LogP |
3.61
|
| tPSA |
74.52
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
11
|
| 重原子数目 |
29
|
| 分子复杂度/Complexity |
484
|
| 定义原子立体中心数目 |
2
|
| SMILES |
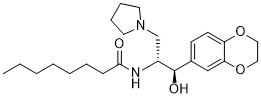
CCCCCCCC(N[C@H](CN1CCCC1)[C@@H](C2=CC=C(OCCO3)C3=C2)O)=O
|
| InChi Key |
N-((1R,2R)-1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-1-hydroxy-3-(pyrrolidin-1-yl)propan-2-yl)octanamide
|
| InChi Code |
FJZZPCZKBUKGGU-AUSIDOKSSA-N
|
| 化学名 |
Genz-99067 GENZ-112638Cerdelga Genz99067 UNIIN0493335P3GENZ 112638Genz 99067 GENZ112638 Eliglustat tartrate eliglustat hemitartrate Eliglustat trade name Cerdelga.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~247.19 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (6.18 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.18 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.18 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03519646 | COMPLETED | Drug: Eliglustat | Gaucher Disease, Type III | National Taiwan University Hospital | 2018-04-23 | Not Applicable |
| NCT06188325 | COMPLETED | Drug: Eliglustat | Gaucher's Disease | Sanofi | 2018-01-01 | Phase 1 |
| NCT06193304 | COMPLETED | Drug: Eliglustat | Gaucher's Disease | Sanofi | 2014-08-25 | Phase 1 |
| NCT02536755 | COMPLETEDWITH RESULTS | Drug: Eliglustat, GZ385660 | Gaucher Disease | Genzyme, a Sanofi Company | 2015-10-27 | Phase 3 |
| NCT02422654 | COMPLETED | Drug: eliglustat | Gaucher Disease | Genzyme, a Sanofi Company | 2015-04 | Phase 1 |