| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|

| 体外研究 (In Vitro) |
Eliglustat酒石酸盐对目标酶具有选择性,疗效良好,IC50为24 nM [1]。通过将 K562 或 B16/F10 细胞与逐渐浓度的 Genz-112638 (0.6-1000 nM) 一起孵育 72 小时,获得剂量依赖性结果。细胞表面的 GM1 和 GM3 水平下降。在 K562 细胞中,GM1 细胞表面呈递抑制的平均 IC50 值为 24 nM(范围 14-34 nM),而在 B16/F10 细胞中,GM3 抑制的平均 IC50 值为 29 nM(范围 12-48 nM)。 1]。
|
|---|---|
| 体内研究 (In Vivo) |
与年龄匹配的对照动物相比,在大量底物积累之前(10周龄)给予药物的小鼠表现出较低水平的葡萄糖神经酰胺以及肝脏、肺和脾脏中较少的戈谢细胞[1]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
A dose of agalsidase alfa in non end stage renal disease patients reaches a Cmax of 3710 ± 855 U/mL with an AUC of 256,958 ± 63,499 min\*U/mL. After nonspecific proteolysis, the amino acids from protein drugs are reused for protein synthesis or further broken down and eliminated by the kidneys. The volume of distribution at steady state in non end stage renal disease patients was approximately 17% of body weight regardless of sex. The clearance for doses of 0.007-0.2 mg/kg were 2.66 mL/min/kg for males and 2.10 mL/min/kg for females. Metabolism / Metabolites Data regarding the metabolism of agalsidase alfa is not readily available. However, protein drugs are expected to be degraded by proteases and other catalytic enzymes to smaller peptides and amino acids. Biological Half-Life The elimination half life was 108 ± 17 minutes for males and 89 ± 28 minutes for females. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Because there is no published experience with eliglustat during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Agalsidase alfa is not expected to be protein bound in circulation. |
| 参考文献 | |
| 其他信息 |
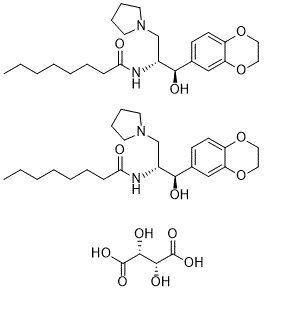
Eliglustat tartrate is a tartrate that is the hemitartrate salt of eliglustat. A ceramide glucosyltransferase inhibitor used (as its tartrate salt) for treatment of Gaucher's disease. It has a role as an EC 2.4.1.80 (ceramide glucosyltransferase) inhibitor. It contains an eliglustat(1+).
Agalsidase alfa is a recombinant human α-galactosidase A similar to [agalsidase beta]. While patients generally do not experience a clinically significant difference in outcomes between the two drugs, some patients may experience greater benefit with agalsidase beta. Use of agalsidase beta has decreased in Europe, in favor of agalsidase alfa, after a contamination event in 2009. Agalsidase alfa was granted EMA approval on 3 August 2001. See also: Eliglustat (has active moiety); Agalsidase Beta (annotation moved to). Drug Indication Agalsidase alfa is indicated in the treatment of Fabry disease. Replagal is indicated for long-term enzyme-replacement therapy in patients with a confirmed diagnosis of Fabry disease (α-galactosidase-A deficiency). Mechanism of Action α-galactosidase A is uptaken by cells via the mannose 6 phosphate receptor. Agalsidase alfa hydrolyzes globotriaosylceramide and other glycosphingolipids that would normally be hydrolyzed by endogenous α-galactosidase A. Preventing the accumulation of glycosphingolipids prevents or reduces the severity of manifestations of Fabry disease such as renal failure, cardiomyopathy, or cerebrovascular events. Pharmacodynamics Agalsidase alfa is a recombinant human α-galactosidase A used as enzyme replacement therapy in the treatment of Fabry disease. It has a long duration of action and a wide therapeutic index. Patients should be counselled regarding the risk of infusion related reactions and hypersensitivity. |
| 分子式 |
2[C23H36N2O4].C4H6O6
|
|---|---|
| 分子量 |
959.173
|
| 精确质量 |
958.551
|
| CAS号 |
928659-70-5
|
| 相关CAS号 |
Eliglustat;491833-29-5;Eliglustat-d15 tartrate;1884556-84-6
|
| PubChem CID |
52918379
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
6.298
|
| tPSA |
264.1
|
| 氢键供体(HBD)数目 |
8
|
| 氢键受体(HBA)数目 |
16
|
| 可旋转键数目(RBC) |
25
|
| 重原子数目 |
68
|
| 分子复杂度/Complexity |
617
|
| 定义原子立体中心数目 |
6
|
| SMILES |
CCCCCCCC(=O)N[C@H](CN1CCCC1)[C@@H](C2=CC3=C(C=C2)OCCO3)O.CCCCCCCC(=O)N[C@H](CN1CCCC1)[C@@H](C2=CC3=C(C=C2)OCCO3)O.[C@@H]([C@H](C(=O)O)O)(C(=O)O)O
|
| InChi Key |
KUBARPMUNHKBIQ-VTHUDJRQSA-N
|
| InChi Code |
InChI=1S/2C23H36N2O4.C4H6O6/c2*1-2-3-4-5-6-9-22(26)24-19(17-25-12-7-8-13-25)23(27)18-10-11-20-21(16-18)29-15-14-28-20;5-1(3(7)8)2(6)4(9)10/h2*10-11,16,19,23,27H,2-9,12-15,17H2,1H3,(H,24,26);1-2,5-6H,(H,7,8)(H,9,10)/t2*19-,23-;1-,2-/m111/s1
|
| 化学名 |
N-[(1R,2R)-1-(2,3-dihydro-1,4-benzodioxin-6-yl)-1-hydroxy-3-pyrrolidin-1-ylpropan-2-yl]octanamide;(2R,3R)-2,3-dihydroxybutanedioic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~104.26 mM)
H2O : ≥ 50 mg/mL (~52.13 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.75 mg/mL (2.87 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 27.5 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.75 mg/mL (2.87 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 27.5mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.75 mg/mL (2.87 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 150 mg/mL (156.39 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.0426 mL | 5.2128 mL | 10.4257 mL | |
| 5 mM | 0.2085 mL | 1.0426 mL | 2.0851 mL | |
| 10 mM | 0.1043 mL | 0.5213 mL | 1.0426 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Biomarkers and Cardiac Imaging Diagnostic Assay for Monitoring Patients With Fabry Disease
CTID: NCT05698901
Phase: Status: Recruiting
Date: 2023-11-18