| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Angiotensin II receptor
|
|---|---|
| 体外研究 (In Vitro) |
Eprosartan (SKF-108566J) 抑制 [125I]AII 与人肝膜(IC50 为 1.7 nM)和大鼠肠系膜动脉膜(IC50 为 1.5 nM)的结合。在兔主动脉平滑肌细胞中,依普罗沙坦对 AII 诱导的细胞内 Ca2+ 水平增加产生浓度依赖性抑制[1]。
|
| 体内研究 (In Vivo) |
在意识正常、血压正常的大鼠中,静脉注射依普罗沙坦(0.01-0.3 mg/kg),在 AII 升压剂量反应曲线中产生剂量依赖性平行变化。对意识正常的血压大鼠十二指肠内或胃内给予依普罗沙坦 (3-10 mg/kg),导致对 AII (250 ng/kg,静脉注射) 的升压反应出现剂量依赖性抑制。在 10 mg/kg,id 下,观察到对 AII 的升压反应的显着抑制持续 3 小时[1]。
Eprosartan(EPRO)是一种血管紧张素受体1型(AT-1)阻断剂,在大鼠局灶性脑缺血引起的缺血性卒中中表现出神经保护活性。本研究旨在阐明EPRO在大鼠中颈动脉闭塞(MCAO)诱导的缺血性卒中中的神经保护作用。56只雄性Wistar大鼠分为四组(每组14只):假手术组、假接受EPRO(60mg/kg/天,po)组、缺血再灌注(IR)组和接受EPRO的IR(60mg/kg/d,po)小组。MCAO导致大鼠运动功能显著受损,同时刺激海马的炎症和凋亡途径。MCAO后,大脑中的AT1受体受到刺激,导致Janus激酶2/信号转导子和转录激活子3信号传导的激活,从而对海马体产生更多的神经炎症环境和破坏性作用。MCAO提高半胱氨酸天冬氨酸蛋白酶-3水平可增强神经元凋亡,与氧化应激生物标志物的神经退行性作用同步。EPRO预处理可对抗大鼠运动损伤,减少海马中的氧化和凋亡介质。EPRO的抗炎活性通过下调核因子κB和肿瘤坏死因子β水平以及(C-X-C基序)配体1信使RNA(mRNA)表达来揭示。此外,该研究证实了EPRO对缺氧诱导因子-1α及其后续炎症介质的独特途径的作用。此外,还观察到小窝蛋白-1 mRNA水平的上调,以及氧化应激标志物水平和脑水肿的降低。因此,EPRO通过减弱氧化、凋亡和炎症途径,在MCAO诱导的大鼠脑缺血中显示出神经保护作用[2]。 |
| 酶活实验 |
在大鼠和人类肾上腺皮质膜中,SK&F 108566分别以9.2和3.9 nM的IC50取代了特异性结合的[125I]AII。SK&F 108566还抑制了[125I]AII与人肝膜(IC50=1.7 nM)和大鼠肠系膜动脉膜(IC50=1.5 nM)的结合[1]。
|
| 细胞实验 |
在兔主动脉平滑肌细胞中,SK&F 108566引起AII诱导的细胞内钙水平升高的浓度依赖性抑制。在兔主动脉环中,SK&F 108566在AII浓度反应曲线中产生平行向右移动,而不影响最大收缩反应。数据的Schild分析得出KB值为0.26nM,斜率与1没有区别,表明竞争拮抗作用。SK&F 108566对兔主动脉对KCl、去甲肾上腺素或内皮素的收缩反应没有影响[1]。
|
| 动物实验 |
In conscious normotensive rats, i.v. administration of SK&F 108566 (0.01-0.3 mg/kg) produced dose-dependent parallel shifts in the AII pressor dose-response curve. Administration of SK&F 108566 (3-10 mg/kg) intraduodenally or intragastrically to conscious normotensive rats resulted in a dose-dependent inhibition of the pressor response to AII (250 ng/kg, i.v.). At 10 mg/kg, i.d., significant inhibition of the pressor response to AII was observed for 3 hr. In this same rat model, SK&F 108566 had no effect on base-line pressure or on the pressor response to norepinephrine or vasopressin. The data demonstrate that SK&F 108566 is a potent, highly selective, competitive nonpeptide AII antagonist.
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absolute bioavailability following a single 300 mg oral dose of eprosartan is approximately 13%. Administering eprosartan with food delays absorption. Eprosartan is excreted in animal milk; it is not known whether eprosartan is excreted in human milk. Plasma protein binding of eprosartan is high (approximately 98%) and constant over the concentration range achieved with therapeutic doses. The pooled population pharmacokinetic analysis from two Phase 3 trials of 299 men and 172 women with mild to moderate hypertension (aged 20 to 93 years) showed that eprosartan exhibited a population mean oral clearance (CL/F) for an average 60-year-old patient of 48.5 L/hr. The population mean steady-state volume of distribution (Vss/F) was 308 L. Eprosartan pharmacokinetics were not influenced by weight, race, gender or severity of hypertension at baseline. Oral clearance was shown to be a linear function of age with CL/F decreasing 0.62 L/hr for every year increase. Eprosartan is eliminated by biliary and renal excretion, primarily as unchanged compound. Less than 2% of an oral dose is excreted in the urine as a glucuronide. There are no active metabolites following oral and intravenous dosing with (14)C eprosartan in human subjects. Eprosartan was the only drug-related compound found in the plasma and feces. Following intravenous (14)C eprosartan, about 61% of the material is recovered in the feces and about 37% in the urine. Following an oral dose of (14)C eprosartan, about 90% is recovered in the feces and about 7% in the urine. Absolute bioavailability following a single 300 mg oral dose of eprosartan is approximately 13%. Eprosartan plasma concentrations peak at 1 to 2 hours after an oral dose in the fasted state. Administering eprosartan with food delays absorption, and causes variable changes (<25%) in Cmax and AUC values which do not appear clinically important. Plasma concentrations of eprosartan increase in a slightly less than dose-proportional manner over the 100 mg to 800 mg dose range. The mean terminal elimination half-life of eprosartan following multiple oral doses of 600 mg was approximately 20 hours. Eprosartan does not significantly accumulate with chronic use. For more Absorption, Distribution and Excretion (Complete) data for EPROSARTAN (6 total), please visit the HSDB record page. Metabolism / Metabolites Eprosartan is not metabolized by the cytochrome P450 system. It is mainly eliminated as unchanged drug. Less than 2% of an oral dose is excreted in the urine as a glucuronide. Following an oral dose of (14)C eprosartan, about 90% is recovered in the feces and about 7% in the urine. Approximately 20% of the radioactivity excreted in the urine was an acyl glucuronide of eprosartan with the remaining 80% being unchanged eprosartan. Biological Half-Life The terminal elimination half-life of eprosartan following oral administration is typically 5 to 9 hours. ... The mean terminal elimination half-life of eprosartan following multiple oral doses of 600 mg was approximately 20 hours. ... After oral administration of eprosartan to healthy volunteers ... the drug's terminal elimination half-life is typically 5-9 hours after oral administration. ... |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Eprosartan is a white to off-white crystalline powder that is formulated into oral tablets. Eprosartan is an angiotensin II type 1 (AT1) receptor antagonist. It is used alone or in combination with other classes of antihypertensive agents in the management of hypertension. HUMAN EXPOSURE AND TOXICITY: The use of eprosartan during pregnancy is contraindicated. While use during the first trimester does not suggest a risk of major anomalies, use during the second and third trimester may cause teratogenicity and severe fetal and neonatal toxicity. Fetal toxic effects may include anuria, oligohydramnios, fetal hypocalvaria, intrauterine growth restriction, premature birth, and patent ductus arteriosus. Anuria-associated oligohydramnios may produce fetal limb contractures, craniofacial deformation, and pulmonary hypoplasia. Severe anuria and hypotension that are resistant to both pressor agents and volume expansion may occur in the newborn following in utero exposure to eprosartan. In human peripheral lymphocytes in vitro, eprosartan was equivocal for clastogenicity with metabolic activation, and was negative without metabolic activation. In the same assay, eprosartan was positive for polyploidy with metabolic activation and equivocal for polyploidy without metabolic activation. ANIMAL STUDIES: Eprosartan was not carcinogenic in dietary restricted rats or ad libitum fed mice dosed at 600 mg and 2000 mg/kg/day, respectively, for up to 2 years. Also, the reproductive performance of male and female rats was unaffected by administration of eprosartan. No adverse effects on in utero or postnatal development and maturation of offspring were observed when eprosartan was administered to pregnant rats at oral doses up to 1000 mg /kg/day. Eprosartan has been shown to produce maternal and fetal toxicities (maternal and fetal mortality, low maternal body weight and food consumption, resorptions, abortions and litter loss) in pregnant rabbits given oral doses. Eprosartan was not mutagenic in vitro in bacteria or mammalian cells (mouse lymphoma assay). Eprosartan also did not cause structural chromosomal damage in vivo (mouse micronucleus assay). Hepatotoxicity Eprosartan has been associated with a low rate of serum aminotransferase elevations ( Likelihood score: E* (Unproved but suspected rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because no information is available on the use of eprosartan during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Plasma protein binding of eprosartan is high (approximately 98%) and constant over the concentration range achieved with therapeutic doses. Interactions Increases in serum lithium concentrations and lithium toxicity have been reported during concomitant administration of lithium with angiotensin II receptor agonists. Monitor serum lithium levels during concomitant use. Do not co-administer aliskiren with Teveten in patients with diabetes. Avoid use of aliskiren with Teveten in patients with renal impairment (GFR <60 mL/min). Dual blockade of the renin-angiotensin system (RAS) with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Most patients receiving the combination of two RAS inhibitors do not obtain any additional benefit compared to monotherapy. In general, avoid combined use of RAS inhibitors. Closely monitor blood pressure, renal function and electrolytes in patients on Teveten and other agents that affect the RAS. Potential pharmacologic interaction (attenuated hypotensive effects) when angiotensin II receptor antagonists are used concomitantly with nonsteroidal anti-inflammatory agents (NSAIAs), including selective cyclooxygenase-2 (COX-2) inhibitors. Possible deterioration of renal function in geriatric, volume-depleted (including those receiving concomitant diuretic therapy), or renally impaired patients; renal function should be monitored periodically in patients receiving concomitant therapy with eprosartan and an NSAIA, including selective COX-2 inhibitors. For more Interactions (Complete) data for EPROSARTAN (7 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Angiotensin II Type 2 Receptor Blockers; Antihypertensive Agents Teveten is indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensives such as diuretics and calcium channel blockers. /Included in US product labeling/ Both angiotensin II receptor antagonists /including eprosartan/ and ACE inhibitors have been shown to slow the rate of progression of renal disease in hypertensive patients with diabetes mellitus and microalbuminuria or overt nephropathy, and use of a drug from either class is recommended in such patients. /NOT included in US product label/ Angiotensin II receptor antagonists /inlcuding eprosartan/ have been used in the management of congestive heart failure. While angiotensin II receptor antagonists appear to share the hemodynamic effects of ACE inhibitors, some experts state that, in the absence of data documenting comparable long-term cardiovascular and/or renal benefits, angiotensin II receptor antagonists should be reserved principally for patients in whom ACE inhibitors are indicated but who are unable to tolerate the drugs (e.g., because of intractable cough or angioedema). /NOT included in US product label/ Drug Warnings /BOXED WARNING/ WARNING: FETAL TOXICITY. When pregnancy is detected, discontinue Teveten as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Teveten as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus. In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examination to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue Teveten, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Neonates with a history of in utero exposure to Teveten: If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function. FDA Pregnancy Risk Category: D /POSITIVE EVIDENCE OF RISK. Studies in humans, or investigational or post-marketing data, have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective./ For more Drug Warnings (Complete) data for EPROSARTAN (17 total), please visit the HSDB record page. Pharmacodynamics Angiotensin II, the principal pressor agent of the renin-angiotensin system, is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme [kininase II]. It is responsible for effects such as vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Eprosartan selectively blocks the binding of angiotensin II to the AT1 receptor, which in turn leads to multiple effects including vasodilation, a reduction in the secretion of vasopressin, and reduction in the production and secretion of aldosterone. The resulting effect is a decrease in blood pressure. |
| 分子式 |
C23H24N2O4S
|
|---|---|
| 分子量 |
424.51
|
| 精确质量 |
424.145
|
| 元素分析 |
C, 65.07; H, 5.70; N, 6.60; O, 15.08; S, 7.55
|
| CAS号 |
133040-01-4
|
| 相关CAS号 |
Eprosartan mesylate; 144143-96-4; Eprosartan-d3; 1185243-70-2
|
| PubChem CID |
5281037
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
660.6±55.0 °C at 760 mmHg
|
| 熔点 |
250-253ºC
|
| 闪点 |
353.3±31.5 °C
|
| 蒸汽压 |
0.0±2.1 mmHg at 25°C
|
| 折射率 |
1.628
|
| LogP |
4.96
|
| tPSA |
120.66
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
618
|
| 定义原子立体中心数目 |
0
|
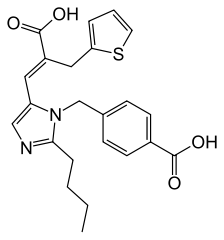
| SMILES |
O=C(O)C1=CC=C(CN2C(/C=C(C(O)=O)\CC3=CC=CS3)=CN=C2CCCC)C=C1
|
| InChi Key |
OROAFUQRIXKEMV-LDADJPATSA-N
|
| InChi Code |
InChI=1S/C23H24N2O4S/c1-2-3-6-21-24-14-19(12-18(23(28)29)13-20-5-4-11-30-20)25(21)15-16-7-9-17(10-8-16)22(26)27/h4-5,7-12,14H,2-3,6,13,15H2,1H3,(H,26,27)(H,28,29)/b18-12+
|
| 化学名 |
4-[[2-butyl-5-[(E)-2-carboxy-3-thiophen-2-ylprop-1-enyl]imidazol-1-yl]methyl]benzoic acid
|
| 别名 |
Regulaten; Futuran; Navixen; Teveten; SKF-108566; SKF108566; Teveten; SKF 108566; SK and F 108566
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~125 mg/mL (~294.5 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.90 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.90 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.90 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3557 mL | 11.7783 mL | 23.5566 mL | |
| 5 mM | 0.4711 mL | 2.3557 mL | 4.7113 mL | |
| 10 mM | 0.2356 mL | 1.1778 mL | 2.3557 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01087749 | Completed | Drug: Propranolol Drug: Eprosartan |
Kidney Disease | University of California, San Francisco |
March 2010 | Phase 1 |
| NCT00160160 | Completed | Drug: eprosartan/HCTZ | Hypertension Type 2 Diabetes |
Solvay Pharmaceuticals | October 2004 | Not Applicable |
| NCT00438945 | Completed | Drug: Eprosartan | Essential Hypertension | Regional Hospital Holstebro | January 2007 | Phase 4 |
| NCT00409903 | Completed | Drug: Eprosartan | Healthy | Regional Hospital Holstebro | November 2006 | Phase 4 |
| NCT01631227 | Completed | Drug: Eprosartan Drug: Placebo Eprosartan |
Essential Hypertension | Abbott | June 2012 | Phase 3 |