| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
VDAC2; VDAC3
|
|---|---|
| 体外研究 (In Vitro) |
异位子宫内膜基质细胞 (EESC) 中的铁死亡由 erematin(10 μM;24 小时)触发,9 小时后,总 ROS 水平上升 [1]。在 EESC 细胞中,红十字蛋白可以缩短线粒体长度并提高其膜密度 [1]。当用红菊酯 (10 μM) 处理 9 小时时,EESC 中铁相关蛋白(包括 FPN(铁输出蛋白))的 mRNA 表达水平较低。另一方面,FPN 的过度表达可以显着预防 Erastin 诱导的 EESC 铁死亡[1]。在 HT-29 结直肠癌细胞中,erematin(10 μM;24 小时)会导致线粒体通透性转换孔 (mPTP) 打开 [2]。 eratin(30 μM;72 小时)可显着抑制 HT-29 结直肠癌细胞的增殖 [2]。控制铁代谢或线粒体脂肪酸代谢的基因参与促红细胞生成素引发铁死亡的生物学机制。包含四肽重复结构域 35、柠檬酸合酶、ATP 合酶 F0 复合体亚基 C3、核糖体蛋白 L8、铁反应元件结合蛋白 2 (IREB2) 和酰基辅酶 A 合成酶家族成员 2 (ACSF2)[3]。
|
| 体内研究 (In Vivo) |
可以用Erastin创建铁死亡诱导的动物模型。在子宫内膜异位症小鼠模型中,Erastin(40 mg/kg;腹腔注射;每 3 天一次,持续 2 周)抑制子宫内膜异位症着床,表明 Erastin 通过诱导铁死亡促进异位病变消退 [1]。在 SCID 小鼠中,eratin(10 mg/kg、30 mg/kg;腹腔注射;每天一次,持续 4 周)抑制 HT-29 异种移植物的生长,其中 30 mg/kg 显示出最大活性 [2]。
|
| 酶活实验 |
Erastin抑制电压依赖性阴离子通道(VDAC2/VDAC3)并加速氧化,导致内源性活性氧的积累。
我们在此评估了erastin(一种电压依赖性阴离子通道(VDAC)结合化合物)潜在的抗结肠癌症活性。我们的体外研究表明,erastin可能通过诱导氧化应激和胱天蛋白酶-9依赖性细胞凋亡,对多种人类结直肠癌癌症细胞系发挥了强大的细胞毒性作用。此外,在erastin处理的癌症细胞中观察到线粒体通透性转变孔(mPTP)开放,这通过VDAC-1和亲环蛋白-D(Cyp-D)结合、线粒体去极化和细胞色素C释放证明。胱天蛋白酶抑制剂、ROS清除剂MnTBAP和mPTP阻断剂(桑格列非林A、环孢菌素A和邦克雷酸),以及shRNA介导的VDAC-1敲低,都显著减弱了erastin诱导的结直肠癌癌症细胞的细胞毒性和凋亡。另一方面,VDAC-1的过度表达增加了erastin诱导的ROS产生、mPTP开放和结直肠癌癌症细胞凋亡。体内研究表明,在严重联合免疫缺陷(SCID)小鼠中,以耐受性良好的剂量腹腔注射erastin可显著抑制HT-29异种移植物的生长。总之,这些结果表明erastin对癌症细胞具有细胞毒性和促凋亡作用。Erastin可以作为一种新型的抗癌症药物被进一步研究。 |
| 细胞实验 |
细胞活力测定[1]
细胞类型:正常子宫内膜基质细胞 (NESC) 和子宫内膜基质细胞 (EESC) 测试浓度: 0、0.5、 0.8、1、1.5、2、2.5、5、10 μM 孵育时间: 24 小时 实验结果:诱导细胞脱离和明显EESC 死亡,但 NESC 不死亡。 细胞凋亡分析[1] 细胞类型:感染表达 FPN cDNA 的腺病毒的 EESC(共孵育 24 小时) 测试浓度: 0、0.5、1.5、2.5、5 和 2.5 μM 孵育时间: 24 小时 实验结果:通过降低总 ROS 和脂质 ROS 水平。并通过腺病毒感染细胞中 FPN 的过度表达而逆转。 |
| 动物实验 |
Animal/Disease Models: Mouse model of endometriosis[1]
Doses: 40 mg/kg Route of Administration: intraperitoneal (ip)injection; once every 3 days for 2 weeks Experimental Results: demonstrated little impact on body weight of mice and hair of mice displayed neat and glossy. decreased the volume of ectopic lesions. Mouse model of endometriosis[1] Ten C57BL/6 female mice (7–8 weeks, weight 20–22 g) were used. Endometriotic lesions were surgically induced by autotransplantation of uterine horns onto the peritoneal wall as previously described. Briefly, uterine horns were removed and opened longitudinally, cut into homogeneous fragments using a 3-mm dermal biopsy punch and then transplanted onto own peritoneal wall of mice by suturing. 17-β-Estradiol-3-benzoate (30 μg/kg) was administered to each postoperative mouse every 3 days for 28 days. At 14 day after operation, endometrial-like lesions were established, and it was time for intervention. They were randomly divided into two groups. In the experimental group, each mouse received erastin (40 mg/kg) by intraperitoneal injection over a 14-day period. In the control group, in place of erastin, soybean oil was used. At 28 days, the mice were sacrificed and we harvested the ectopic tissues. The volumes of ectopic lesions were measured and analyzed as previously described (Zhao et al., 2015). |
| 参考文献 | |
| 其他信息 |
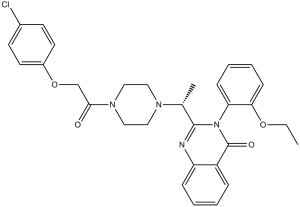
Erastin is a member of the class of quinazolines that is quinazolin-4(3H)-one in which the hydrogens at positions 2 and 3 are replaced by 1-{4-[(4-chlorophenoxy)acetyl]piperazin-1-yl}ethyl and 2-ethoxyphenyl groups, respectively. It is an inhibitor of voltage-dependent anion-selective channels (VDAC2 and VDAC3) and a potent ferroptosis inducer. It has a role as a ferroptosis inducer, an antineoplastic agent and a voltage-dependent anion channel inhibitor. It is a member of quinazolines, a member of monochlorobenzenes, an aromatic ether, a N-acylpiperazine, a N-alkylpiperazine, a diether and a tertiary carboxamide.
Study question: Could erastin activate ferroptosis to regress endometriotic lesions? Summary answer: Erastin could induce ferroptosis to regress endometriotic lesions in endometriosis. What is known already: Ectopic endometrial stromal cells (EESCs) are in an iron overloading microenvironment and tend to be more sensitive to oxidative damage. The feature of erastin-induced ferroptosis is iron-dependent accumulation of lethal lipid reactive oxygen species (ROS). Study design, size, duration: Eleven patients without endometriosis and 21 patients with endometriosis were recruited in this study. Primary normal and ectopic endometrial stromal cells were isolated, cultured and subjected to various treatments. The in vivo study involved 10 C57BL/6 female mice to establish the model of endometriosis. Participants/materials, setting, methods: The markers of ferroptosis were assessed by cell viability, lipid peroxidation level and morphological changes. The cell viability was measured by colorimetric method, lipid peroxidation levels were measured by flow cytometry, and morphological changes were observed by transmission electron microscopy. Immunohistochemistry and western blot were used to detect ferroportin (FPN) expression. Prussian blue staining and immunofluorescent microscopy of catalytic ferrous iron were semi-quantified the levels of iron. Adenovirus-mediated overexpression and siRNA-mediated knockdown were used to investigate the role of FPN on erastin-induced ferroptosis in EESCs. Main results and the role of chance: EESCs were more susceptible to erastin treatment, compared to normal endometrial stromal cells (NESCs) (P<0.05). Treatment of cultured EESCs with erastin dramatically increased the total ROS level (P<0.05, versus control), lipid ROS level (P<0.05, versus NESCs) and intracellular iron level (P<0.05, versus NESCs). The cytotoxicity of erastin could be attenuated by iron chelator, deferoxamine (DFO), and ferroptosis inhibitors, ferrostatin-1 and liproxstatin-1, (P<0.05, versus erastin) in EESCs. In EESCs with erastin treatment, shorter and condensed mitochondria were observed by electron microscopy. These findings together suggest that erastin is capable to induce EESC death by ferroptosis. However, the influence of erastin on NESCs was slight. The process of erastin-induced ferroptosis in EESCs accompanied iron accumulation and decreased FPN expression. The overexpression of FPN ablated erastin-induced ferroptosis in EESCs. In addition, knockdown of FPN accelerated erastin-induced ferroptosis in EESCs. In a mouse model of endometriosis, we found ectopic lesions were regressed after erastin administration. Large scale data: N/A. Limitations, reasons for caution: This study was mainly conducted in primary human endometrial stromal cells. Therefore, the function of FPN in vivo need to be further investigated. Wider implications of the findings: Our findings reveal that erastin may serve as a potential therapeutic treatment for endometriosis. Study funding/competing interest(s): This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. The authors declare no conflict of interest.[1] We here evaluated the potential anti-colorectal cancer activity by erastin, a voltage-dependent anion channel (VDAC)-binding compound. Our in vitro studies showed that erastin exerted potent cytotoxic effects against multiple human colorectal cancer cell lines, possibly via inducing oxidative stress and caspase-9 dependent cell apoptosis. Further, mitochondrial permeability transition pore (mPTP) opening was observed in erastin-treated cancer cells, which was evidenced by VDAC-1 and cyclophilin-D (Cyp-D) association, mitochondrial depolarization, and cytochrome C release. Caspase inhibitors, the ROS scavenger MnTBAP, and mPTP blockers (sanglifehrin A, cyclosporin A and bongkrekic acid), as well as shRNA-mediated knockdown of VDAC-1, all significantly attenuated erastin-induced cytotoxicity and apoptosis in colorectal cancer cells. On the other hand, over-expression of VDAC-1 augmented erastin-induced ROS production, mPTP opening, and colorectal cancer cell apoptosis. In vivo studies showed that intraperitoneal injection of erastin at well-tolerated doses dramatically inhibited HT-29 xenograft growth in severe combined immunodeficient (SCID) mice. Together, these results demonstrate that erastin is cytotoxic and pro-apoptotic to colorectal cancer cells. Erastin may be further investigated as a novel anti-colorectal cancer agent. [2] Piperlongumine, a natural alkaloid substance extracted from the fruit of the long pepper (Piper longum Linn.), is known to inhibit the cytosolic thioredoxin reductase (TXNRD1 or TrxR1) and selectively kill cancer cells. However, the details and mechanism of the inhibition by piperlongumine against TXNRD1 remain unclear. In this study, based on the classical DTNB reducing assay, irreversible inhibition of recombinant TXNRD1 by piperlongumine was found and showed an apparent kinact value of 0.206 × 10-3 µM-1 min-1. Meanwhile, compared with the wild-type TXNRD1 (-GCUG), the UGA-truncated form (-GC) of TXNRD1 was resistant to piperlongumine, suggesting the preferential target of piperlongumine is the selenol (-SeH) at the C-terminal redox motif of the enzyme. Interestingly, the high concentration of piperlongumine-inhibited TXNRD1 showed that its Sec-dependent activity is decayed but its intrinsic NADPH oxidase activity is retained. Furthermore, piperlongumine did not induce ferroptosis in HCT116 cells at 10 µM, whereas significantly promoted erastin-induced lipid oxidation, which could be alleviated by supplying glutathione (GSH) or N-acetyl L-cysteine (NAC). However, restricting GSH synthesis by inhibiting glutaminase (GLS) using the small molecule inhibitor CB-839 only slightly enhanced erastin-induced cell death. Taken together, this study elucidates the molecular mechanism of the antitumor capacity of piperlongumine by targeting TXNRD1 and reveals the potential possibility of inhibiting TXNRD1 to strengthen cancer cells' ferroptosis. [5] |
| 分子式 |
C30H31CLN4O4
|
|
|---|---|---|
| 分子量 |
547.04
|
|
| 精确质量 |
546.203
|
|
| 元素分析 |
C, 65.87; H, 5.71; Cl, 6.48; N, 10.24; O, 11.70
|
|
| CAS号 |
571203-78-6
|
|
| 相关CAS号 |
|
|
| PubChem CID |
11214940
|
|
| 外观&性状 |
White to off-white solid
|
|
| 密度 |
1.3±0.1 g/cm3
|
|
| 沸点 |
721.9±70.0 °C at 760 mmHg
|
|
| 闪点 |
390.4±35.7 °C
|
|
| 蒸汽压 |
0.0±2.3 mmHg at 25°C
|
|
| 折射率 |
1.634
|
|
| LogP |
4.75
|
|
| tPSA |
76.9
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
8
|
|
| 重原子数目 |
39
|
|
| 分子复杂度/Complexity |
871
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
ClC1C([H])=C([H])C(=C([H])C=1[H])OC([H])([H])C(N1C([H])([H])C([H])([H])N(C([H])([H])C1([H])[H])C([H])(C([H])([H])[H])C1=NC2=C([H])C([H])=C([H])C([H])=C2C(N1C1=C([H])C([H])=C([H])C([H])=C1OC([H])([H])C([H])([H])[H])=O)=O
|
|
| InChi Key |
BKQFRNYHFIQEKN-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C30H31ClN4O4/c1-3-38-27-11-7-6-10-26(27)35-29(32-25-9-5-4-8-24(25)30(35)37)21(2)33-16-18-34(19-17-33)28(36)20-39-23-14-12-22(31)13-15-23/h4-15,21H,3,16-20H2,1-2H3
|
|
| 化学名 |
2-(1-(4-(2-(4-chlorophenoxy)acetyl)piperazin-1-yl)ethyl)-3-(2-ethoxyphenyl)quinazolin-4(3H)-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 该产品在溶液状态不稳定,请现配现用。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.25 mg/mL (2.29 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 12.5 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 配方 2 中的溶解度: ≥ 1 mg/mL (1.83 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 10.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: 5% DMSO+corn oil: 2.5mg/mL 配方 4 中的溶解度: 5 mg/mL (9.14 mM) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8280 mL | 9.1401 mL | 18.2802 mL | |
| 5 mM | 0.3656 mL | 1.8280 mL | 3.6560 mL | |
| 10 mM | 0.1828 mL | 0.9140 mL | 1.8280 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。