| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |

| 靶点 |
Macrolide antibiotic; Antiviral; HIV-1
|
|---|---|
| 体外研究 (In Vitro) |
巨噬细胞(MPhis)是HIV-1的主要来源,尤其是在肺结核患者中。有些MPhis是允许的,也有限制HIV-1的。造血细胞激酶(Hck)活性的调节和CCAAT增强子结合蛋白β(C/EBPbeta)亚型的选择性表达大大有助于确定MPhis对HIV-1的不同易感性。抗性可归因于Hck的表达减少和C/EBPbeta的抑制性小亚型的表达增加。红霉素A(EMA)的衍生物EM201和EM703在转录后和翻译水平上抑制HIV-1在组织MPhis中的复制。我们证明,EM201和EM703通过下调Hck和诱导C/EBPbeta的小亚型,将组织MPhis从HIV-1易感转化为HIV-1抗性。这些药物抑制p38MAPK的激活,该激活仅在易感组织MPhis中表达。活化的CD4(+)T细胞通过ERK1/2的活化下调C/EBPbeta的小亚型,刺激HIV-1抗性MPhis中的病毒复制。EM201和EM703可以抑制MAPK的激活,并抑制CD4(+)T细胞和MPhis相互作用时产生的病毒复制的爆发。这些EM衍生物可能对抑制HIV-1感染患者淋巴网状系统中残留的HIV-1非常有益,并为未来治疗艾滋病患者创造新的抗HIV药物提供了巨大的前景[4]。
|
| 体内研究 (In Vivo) |
在瑞典,有几篇报道称,母马在其小马驹口服红霉素和利福平治疗马红球菌肺炎时,患上了急性结肠炎。在这项研究中,6匹成年马被单独或联合口服低剂量的这些抗生素。在红霉素给药后3天内,在一例与利福平联合用药的病例中,2匹马出现严重结肠炎(一例死亡)。艰难梭菌是从其中一匹马身上分离出来的,而另一匹马没有分离出特定的病原体。在急性结肠炎中,两匹马的血液参数都有典型的变化。艰难梭菌也从第三匹马的粪便中分离出来,第三匹马服用了更低剂量的红霉素和利福平。这匹马出现了非常轻微的临床症状,并自行康复。在第四匹马中,仅给予红霉素,分离出非常高数量的产气荚膜梭菌。服用利福平的马没有出现任何临床症状,粪便菌群也没有重大变化。总之,已经证明低剂量的乙基琥珀酸红霉素可以诱导马的严重结肠炎,并与肠道菌群的主要变化有关。艰难梭菌已被证明是抗生素诱导的急性结肠炎的潜在病因[5]。
|
| 酶活实验 |
HIV-1毒株与感染。从感染M-MΦs的HIV-1菌株的培养上清液中收集嗜M型HIV-1菌株HIV-BaL作为病毒资源。将Mo衍生的MΦs与DNase处理的病毒上清液的100 pg/ml p24抗原在37°C下孵育2小时(p24,50%组织培养感染剂量(TCID50)和感染多重性(MOI)分别为50 ng/ml、~3000和0.05),然后在含有10%FCS和CSF的RPMI培养基1640中培养。如有必要,用100μM AZT在4°C下预处理病毒接种物2小时。每3-4天加入一次含有CSF的新鲜培养基(体积的20%)。使用热灭活病毒(1小时,56°C)作为阴性对照。通过使用两种抗体的组合的ELISA连续测量上清液中的p24抗原来测定病毒产生;抗gag-p24单克隆抗体(Nu24)和过氧化物酶标记的10B5,或用于低水平p24抗原的高亲和力检测的RETRO-TEK HIV-1 p24抗原ELISA试剂盒[4]。
|
| 细胞实验 |
HIV-1感染的GM-MΦs与活化的CD4+T细胞的共培养。使用具有抗CD4 mAb包被的微珠的MACS从CD14−PBMC中阳性分离CD4+T细胞。所选人群CD3和CD4阳性率>93%。通过用PHA刺激制备活化的CD4+T细胞,并用IL-2(30单位/ml)培养。将GM-MΦs与DNase处理的病毒上清液的100 pg/ml p24抗原在37°C下孵育2小时,洗涤两次,然后在IL-2存在下与活化的CD4+T细胞共培养[4]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
ERYTHROMYCIN ETHYLSUCCINATE IS PARTIALLY DISSOCIATED IN INTESTINE; BOTH ERYTHROMYCIN & UNDISSOCIATED ESTER ARE ABSORBED &, IN THE BLOOD, THE ESTER IS HYDROLYZED TO RELEASE FREE ERYTHROMYCIN. ERYTHROMYCIN ETHYLSUCCINATE...IS ADEQUATELY ABSORBED FOLLOWING ORAL ADMINISTRATION, PARTICULARLY WHEN THE STOMACH IS EMPTY. PEAK CONCENTRATIONS IN PLASMA ARE 1.5 UG/ML (0.5 UG/ML OF BASE) 1 TO 2 HR AFTER ADMINISTRATION OF A 500 MG TABLET. IN PATIENTS, MEAN BILE LEVELS OF ERYTHROMYCIN WERE APPROXIMATELY 10 TIMES HIGHER THAN CORRESPONDING SERUM CONCN 1 HR AFTER IV (ERYTHROMYCIN LACTOBIONATE) & IM (ERYTHROMYCIN SUCCINATE) INJECTION. IN HEALTHY ADULT SUBJECTS & IN ADULT PATIENTS WITH BRONCHIAL INFECTIONS, PHARMACOKINETICS OF VARIOUS FORMULATIONS OF ERYTHROMYCIN WERE STUDIED. ERYTHROMYCIN ETHYLSUCCINATE WAS BETTER THAN ERYTHROMYCIN STEARATE FOR ORAL TREATMENT IN THAT IT WAS RAPIDLY & CONSISTENTLY ABSORBED. |
| 参考文献 |
[1]. Erythromycin. Med Clin North Am. 1982 Jan;66(1):79-89. [4]. Erythromycin derivatives inhibit HIV-1 replication in macrophages through modulation of MAPK activity to induce small isoforms of C/EBPbeta. Proc Natl Acad Sci U S A. 2008 Aug 26;105(34):12509-14.[5]. The association of erythromycin ethylsuccinate with acute colitis in horses in Sweden. Equine Vet J. 1997 Jul;29(4):314-8. |
| 其他信息 |
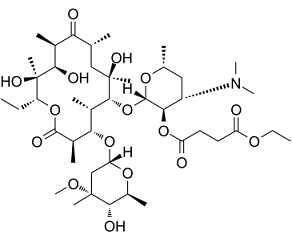
Erythromycin ethylsuccinate is a erythromycin derivative that is erythromycin A in which the hydroxy group at position 3R is substituted by a (4-ethoxy-4-oxobutanoyl)oxy group. It is used for the treatment of a wide variety of bacterial infections. It is a succinate ester, a cyclic ketone, an erythromycin derivative and an ethyl ester. It is functionally related to an erythromycin A.
Erythromycin Ethylsuccinate is the ethylsuccinate salt form of erythromycin, a broad-spectrum, topical macrolide antibiotic with antibacterial activity. Erythromycin ethylsuccinate diffuses through the bacterial cell membrane and reversibly binds to the 50S subunit of the bacterial ribosome. This prevents bacterial protein synthesis. Erythromycin ethylsuccinate may be bacteriostatic or bactericidal in action, depending on the concentration of the drug at the site of infection and the susceptibility of the organism involved. A macrolide antibiotic, produced by Streptomyces erythreus. This compound is an ester of erythromycin base and succinic acid. It acts primarily as a bacteriostatic agent. In sensitive organisms, it inhibits protein synthesis by binding to 50S ribosomal subunits. This binding process inhibits peptidyl transferase activity and interferes with translocation of amino acids during translation and assembly of proteins. See also: Erythromycin (has active moiety); Erythromycin ethylsuccinate; sulfisoxazole acetyl (component of). Therapeutic Uses Antibiotics, Macrolide; Enzyme Inhibitors; Gastrointestinal Agents; Protein Synthesis Inhibitors RELATIVELY NONIRRITATING...& THUS IS WELL SUITED TO IM INJECTION. ...ITS ACTIONS & USES ARE ESSENTIALLY THOSE OF ERYTHROMYCIN...INTO WHICH IT IS CONVERTED IN THE BODY. ERYTHROMYCIN IS...ANTIMICROBIAL... ERYTHROMYCIN IS.../ACTIVE/ AGAINST MOST GRAM-POSITIVE BACTERIA, MANY ANAEROBES...& LEGIONNAIRE'S BACILLUS. /ERYTHROMYCIN/ ERYTHROMYCIN MAY BE...BACTERIOSTATIC OR BACTERICIDAL DEPENDING ON NATURE OF MICROORGANISM & CONCN OF DRUG. ...MOST EFFECTIVE IN VITRO AGAINST GRAM-POSITIVE COCCI SUCH AS STAPHYLOCOCCUS AUREUS (PENICILLIN G SENSITIVE OR RESISTANT), GROUP A STREPTOCOCCI, ENTEROCOCCI & PNEUMOCOCCI; MANY GRAM-POSITIVE BACILLI... /ERYTHROMYCIN/ A REVIEW OF THE ANTIMICROBIAL SPECTRUM, PHARMACOLOGY & THERAPEUTIC USE OF ERYTHROMYCIN & ITS DERIVATIVES. (REF 24). |
| 分子式 |
C43H75NO16
|
|---|---|
| 分子量 |
862.0527
|
| 精确质量 |
861.508
|
| 元素分析 |
C, 59.91; H, 8.77; N, 1.62; O, 29.69
|
| CAS号 |
1264-62-6
|
| 相关CAS号 |
Erythromycin;114-07-8;Erythromycin ethylsuccinate-13C,d3
|
| PubChem CID |
443953
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
874.1±65.0 °C at 760 mmHg
|
| 熔点 |
219-224ºC
|
| 闪点 |
482.4±34.3 °C
|
| 蒸汽压 |
0.0±0.6 mmHg at 25°C
|
| 折射率 |
1.529
|
| 来源 |
Saccharopolyspora erythraea
|
| LogP |
4.1
|
| tPSA |
226.28
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
17
|
| 可旋转键数目(RBC) |
14
|
| 重原子数目 |
60
|
| 分子复杂度/Complexity |
1450
|
| 定义原子立体中心数目 |
18
|
| SMILES |
O([C@@]1([H])[C@@]([H])([C@]([H])(C([H])([H])[C@@]([H])(C([H])([H])[H])O1)N(C([H])([H])[H])C([H])([H])[H])OC(C([H])([H])C([H])([H])C(=O)OC([H])([H])C([H])([H])[H])=O)[C@@]1([H])[C@@](C([H])([H])[H])(C([H])([H])[C@@]([H])(C([H])([H])[H])C([C@]([H])(C([H])([H])[H])[C@]([H])([C@@](C([H])([H])[H])([C@@]([H])(C([H])([H])C([H])([H])[H])OC([C@]([H])(C([H])([H])[H])[C@]([H])([C@]1([H])C([H])([H])[H])O[C@@]1([H])C([H])([H])[C@](C([H])([H])[H])([C@]([H])([C@]([H])(C([H])([H])[H])O1)O[H])OC([H])([H])[H])=O)O[H])O[H])=O)O[H]
|
| InChi Key |
NSYZCCDSJNWWJL-YXOIYICCSA-N
|
| InChi Code |
InChI=1S/C43H75NO16/c1-15-29-43(11,52)36(48)24(5)33(47)22(3)20-41(9,51)38(25(6)34(26(7)39(50)57-29)59-32-21-42(10,53-14)37(49)27(8)56-32)60-40-35(28(44(12)13)19-23(4)55-40)58-31(46)18-17-30(45)54-16-2/h22-29,32,34-38,40,48-49,51-52H,15-21H2,1-14H3/t22-,23-,24+,25+,26-,27+,28+,29-,32+,34+,35-,36-,37+,38-,40+,41-,42-,43-/m1/s1
|
| 化学名 |
(2S,3R,4S,6R)-4-(dimethylamino)-2-(((3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-14-ethyl-7,12,13-trihydroxy-4-(((2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-3,5,7,9,11,13-hexamethyl-2,10-dioxooxacyclotetradecan-6-yl)oxy)-6-methyltetrahydro-2H-pyran-3-yl
ethyl succinate
|
| 别名 |
Erythromycin ethylsuccinate; E-Mycin E; E.E.S; Wyamycin; Wyamycin E;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 50 mg/mL (~58.00 mM)
Ethanol :≥ 33.33 mg/mL (~38.66 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (2.90 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (2.90 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.1600 mL | 5.8001 mL | 11.6003 mL | |
| 5 mM | 0.2320 mL | 1.1600 mL | 2.3201 mL | |
| 10 mM | 0.1160 mL | 0.5800 mL | 1.1600 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。