| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| 100g |
|
||
| Other Sizes |
| 靶点 |
Macrolide antibiotic
|
|---|---|
| 体外研究 (In Vitro) |
当红霉素存在时,恶性疟原虫不能生长;其IC50和IC90值分别为58.2 μM和104.0 μM[1]。
红霉素(10 μM、100 μM;24 h、72 h)具有抗炎和抗氧化特性。它还抑制 4-HNE (p<0.01) 和 8-OHdG (p<0.01) 的积累,并显着降低 TNF-α (p<0.01) 和 Iba-1 (p<0.01) 的表达[4]。 |
| 体内研究 (In Vivo) |
红霉素(胃插管;0.1-50 mg/kg;30-120 天)在接受 5 mg/kg 剂量后可减缓肿瘤的生长并增加小鼠的存活时间。当剂量为 50 mg/kg 时kg,红霉素(胃插管;5 mg/kg)可将荷瘤小鼠的平均生存时间缩短 4-5 天。然而,即使在接种后 120 天,它仍能保护小鼠存活。[3]。单次注射红霉素(ih;50 mg/kg)可保护大鼠模型免受脑缺血再灌注损伤[4]。
|
| 酶活实验 |
红霉素抑制恶性疟原虫的生长,IC50和IC90值分别为58.2+/-7.7微M和104.0+/-10.8微M。比较了抗疟药物与阿奇霉素或红霉素联合使用对恶性疟原虫K1的活性。氯喹与阿奇霉素或红霉素的组合在体外显示出对寄生虫生长的协同作用。奎宁-阿奇霉素和奎宁-红霉素的组合显示出增强作用。在甲氟喹-阿奇霉素和甲氟喹红霉素组合中观察到相加效应。焦萘啶与阿奇霉素或红霉素联合使用也产生了类似的结果。然而,青蒿琥酯-阿奇霉素和青蒿琥酸酯-红霉素组合具有拮抗作用。体外数据表明,阿奇霉素和红霉素与氯喹和奎宁联合使用具有临床实用性。抗氯喹恶性疟原虫在全球范围内的传播可能会抑制在耐药地区使用氯喹阿奇霉素和氯喹红霉素治疗疟疾患者的能力。对抗多重耐药恶性疟原虫的最佳药物组合是奎宁-阿奇霉素和奎宁-红霉素[4]。
|
| 细胞实验 |
细胞系:胚胎原代皮质神经元(源自17日龄Sprague-Dawley大鼠大脑皮层)
浓度: 10, 100 μM 培养时间: 24, 72小时 结果: 培养量增加三小时缺氧葡萄糖 (OGD) 后神经元细胞的体外活力。 |
| 动物实验 |
Animal Model: Six-week-old female ddY mice with EAC cellsor six-week-old CDF mice with P388 cells[3]
Dosage: 0.1 mg/kg; 0.5 mg/kg; 10 mg/kg; 30 mg/kg; 50 mg/kg
Administration: Gastric intubation; 30-120 days
Result: reduced tumor growth and extended the mice's mean survival time (5 mg/kg); in contrast, the 50 mg/kg dose caused the MST in tumor-bearing mice to be shorter.
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Orally administered erythromycin is readily absorbed. Food intake does not appear to exert effects on serum concentrations of erythromycin. Some interindividual variation exists in terms of erythromycin absorption, which may impact absorption to varying degrees. The Cmax of erythromycin is 1.8 mcg/L and the Tmax is 1.2 hours. The serum AUC of erythromycin after the administration of a 500mg oral dose was 7.3±3.9 mg.h/l in one pharmacokinetic study. Erythromycin is well known for a bioavailability that is variable (18-45%) after oral administration and its susceptibility to broken down under acidic conditions. In patients with normal liver function, erythromycin concentrates in the liver and is then excreted in the bile.Under 5% of the orally administered dose of erythromycin is found excreted in the urine. A high percentage of absorbed erythromycin is not accounted for, but is likely metabolized. Erythromycin is found in most body fluids and accumulates in leucocytes and inflammatory liquid. Spinal fluid concentrations of erythromycin are low, however, the diffusion of erythromycin through the blood-brain barrier increases in meningitis, likely due to the presence of inflamed tissues which are easily penetrated. Erythromycin crosses the placenta. The clearance of erythromycin in healthy subjects was 0.53 ± 0.13 l/h/kg after a 125mg intravenous dose. In a clinical study of healthy patients and patients with liver cirrhosis, clearance of erythromycin was significantly reduced in those with severe liver cirrhosis. The clearance in cirrhotic patients was 42.2 ± 10.1 l h–1 versus 113.2 ± 44.2 l h-1 in healthy patients. Absorption of orally administered erythromycins occurs mainly in the duodenum. The bioavailability of the drugs is variable and depends on several factors including the particular erythromycin derivative, the formulation of the dosage form administered, acid stability of the derivative, presence of food in the GI tract, and gastric emptying time. Erythromycin is rather slowly absorbed after oral administration. peak serum concentrations ranged from 0.1 to 4.8 ug/mL according to the form and the coating of erythromycin administered. The oral absorption is less that 50% and erythromycin is degraded by gastric acid. It is absorbed in the small intestine (mainly in duodenum for humans) as erythromycin base. Erythromycin diffuses readily into intracellular fluids, achieving antibacterial activity in essentially all sites except the brain and CSF. Erythromycin penetrates into prostatic fluid, achieving concentrations approximately 40% of those in plasma. Concentrations in middle ear exudate reach only 50% of serum concentrations and thus may be inadequate for the treatment of otitis media caused by H. influenzae. Protein binding is approximately 70% to 80% for erythromycin base and even higher, 96%, for the estolate. Erythromycin traverses the placenta, and drug concentrations in fetal plasma are about 5% to 20% of those in the maternal circulation. Concentrations in breast milk are 50% of those in serum. In an in vitro model using human skin, erythromycin was absorbed into the stratum corneum following topical application of 10-20 mg of the drug in a vehicle containing dimethylacetamide and 95% alcohol. The drug does not appear to be absorbed systemically following twice daily application of a 2% solution of the drug in a vehicle containing 77% alcohol and polyethylene glycol and acetone. It is not known if erythromycin is absorbed from intact or denuded skin, wounds, or mucous membranes following topical application of an ointment containing the drug. For more Absorption, Distribution and Excretion (Complete) data for Erythromycin (13 total), please visit the HSDB record page. PEAK CONCN IN PLASMA...0.3-0.5 UG/ML 4 HR AFTER ORAL ADMIN OF 250 MG OF BASE & ARE 0.3-1.9 UG/ML AFTER...500-MG TABLET. VARIOUS ESTERS OF ERYTHROMYCIN HAVE BEEN PREPARED TO...IMPROVE STABILITY & FACILITATE ABSORPTION. ...CONCN OF ERYTHROMYCIN IN PLASMA ARE LITTLE DIFFERENT IF STEARATE IS GIVEN ORALLY. ...DIFFUSES READILY INTO INTRACELLULAR FLUIDS, & ANTIBACTERIAL ACTIVITY... ACHIEVED AT...ALL SITES EXCEPT BRAIN & CSF. ...ONE OF FEW ANTIBIOTICS THAT PENETRATES INTO PROSTATIC FLUID, CONCN ARE APPROX 40% OF...PLASMA. EXTENT OF BINDING...TO PLASMA PROTEINS VARIES...PROBABLY EXCEEDS 70% IN ALL.../FORMS OF DRUG/. /ERYTHROMYCIN/ ERYTHROMYCIN BASE IS ADEQUATELY ABSORBED FROM UPPER PART OF SMALL INTESTINE; IT IS INACTIVATED BY GASTRIC JUICE... FOOD IN STOMACH DELAYS ITS ULTIMATE ABSORPTION. /ERYTHROMYCIN/ ERYTHROMYCIN TRAVERSES PLACENTAL BARRIER; & CONCN OF DRUG IN FETAL PLASMA ARE ABOUT 5-20% OF THOSE IN MATERNAL CIRCULATION. /ERYTHROMYCIN/ For more Absorption, Distribution and Excretion (Complete) data for ERYTHROMYCIN STEARATE (11 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic first-pass metabolism contributes significantly to erythromycin metabolism after an oral dose. Erythromycin is partially metabolized by CYP3A4 enzyme to N-desmethylerythromycin. Erythromycin is also hydrolyzed to _anhydro_ forms (anhydroerythromycin [AHE] and other metabolites), and this process is promoted by acidic conditions. AHE is inactive against microbes but inhibits hepatic drug oxidation and is therefore considered to be an important contributor to erythromycin drug-drug interactions. Twenty hours after an oral administration of 10 mg erythromycin to rats, about 37-43% of the administered radioactivity was recovered in the intestinal tract plus feces, 27.2 to 36.1% in the urine, 21-29% in the expired air. It was rapidly metabolized in the liver, mainly through demethylation process, and excreted in the bile as des-N-methyl-erythromycin, the major metabolite present only in the bile and in the intestinal contents of rats. The isotropic methyl group was eliminated in the expired air as CO2. IT IS HYDROLYZED IN SMALL INTESTINE & IN TISSUES TO YIELD ERYTHROMYCIN. Hepatic. Extensively metabolized - after oral administration, less than 5% of the administered dose can be recovered in the active form in the urine. Erythromycin is partially metabolized by CYP3A4 resulting in numerous drug interactions. Half Life: 0.8 - 3 hours Biological Half-Life The elimination half-life of oral erythromycin was 3.5 hours according to one study and ranged between 2.4-3.1 hours in another study. Repetitive dosing of erythromycin leads to increased elimination half-life. ... The serum elimination half-life of erythromycin is approximately 1.6 hours. The serum half-life in normal subjects is 2 hours and in anuric subjects, 4-6 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Erythromycin acts by penetrating the bacterial cell membrane and reversibly binding to the 50 S subunit of bacterial ribosomes or near the “P” or donor site so that binding of tRNA (transfer RNA) to the donor site is blocked. Translocation of peptides from the “A” or acceptor site to the “P” or donor site is prevented, and subsequent protein synthesis is inhibited. Erythromycin is effective only against actively dividing organisms. The exact mechanism by which erythmromycin reduces lesions of acne vulgaris is not fully known: however, the effect appears to be due in part to the antibacterial activity of the drug. Interactions Erythromycin is metabolized by CYP3A and concomitant use with drugs that inhibit the CYP3A isoenzyme may result in increased erythromycin plasma concentrations. There is some evidence that concomitant use of oral erythromycin with drugs that inhibit CYP3A (i.e., fluconazole, ketoconazole, itraconazole, diltiazem, verapamil) is associated with an increased incidence of sudden death from cardiac causes, presumably because of increased plasma erythromycin concentrations resulting in an increased risk of QT prolongation (a dose-associated effect of erythromycin) and serious ventricular arrhythmias. Therefore, it has been suggested that concomitant use of erythromycin and drugs that are potent inhibitors of CYP3A should be avoided. Erythromycin may interact with astemizole and terfenadine (both drugs no longer commercially available in the US), resulting in potentially serious adverse cardiovascular effects. Some evidence indicates that erythromycin may alter the metabolism of astemizole and terfenadine, probably via inhibition of the cytochrome P-450 microsomal enzyme system. While erythromycin has been shown to decrease markedly the clearance of the active carboxylic acid metabolite of terfenadine, the effect of the macrolide on unchanged terfenadine concentrations has not been fully elucidated, but appears to show interindividual variation. In studies in extensive metabolizers of dextromethorphan or debrisoquin, erythromycin markedly impaired clearance of the active metabolite of terfenadine in all such individuals but produced measurable effects on unchanged terfenadine in only one-third of these individuals. In addition, erythromycin is known to inhibit the enzyme system responsible for astemizole's metabolism. Prolongation of the QT interval and ventricular tachycardia, including torsades de pointes, have been reported in some patients receiving astemizole or terfenadine concomitantly with erythromycin or the structurally related macrolide troleandomycin (no longer commercially available in the US). Rarely, cardiac arrest and death have been reported in patients receiving erythromycin and terfenadine concomitantly. Therefore, when terfenadine and astemizole were commercially available in the US, these antihistamines were contraindicated in patients receiving erythromycin, clarithromycin, or troleandomycin. In addition, concomitant administration of astemizole or terfenadine and azithromycin also was not recommended, although limited data suggested that azithromycin did not alter the metabolism of terfenadine. Although in vitro studies have shown varying degrees of additive or synergistic effects against some organisms when erythromycin was used in conjunction with penicillins, streptomycin, sulfonamides, rifampin, or chloramphenicol, the clinical importance of these reports has not been established. Antagonism of bactericidal activity has been observed between erythromycin and clindamycin in vitro. In addition, antagonism has been reported when a bacteriostatic drug was administered with a bactericidal drug, but antagonism has not been convincingly documented clinically. Concomitant use of erythromycin in patients receiving high dosage of theophylline has resulted in decreased clearance of theophylline, elevated serum theophylline concentrations, and a prolonged serum half-life of the bronchodilator. An interaction may be most likely to occur in patients receiving an erythromycin dosage greater than 1.5 g daily for more than 5 days. Patients receiving theophylline should be closely monitored for signs of theophylline toxicity when erythromycin is administered concomitantly; serum theophylline concentrations should be monitored and dosage of the bronchodilator reduced if indicated. Although further study is needed and the clinical importance has not been determined to date, there is some evidence that concomitant administration of erythromycin and theophylline can also result in decreased serum erythromycin concentrations and subtherapeutic concentrations of erythromycin may occur. For more Interactions (Complete) data for Erythromycin (22 total), please visit the HSDB record page. A 77-YR-OLD WOMAN IS REPORTEDLY MAINTAINED ON 7.5 MG OF WARFARIN DAILY IN WHOM THE ADMIN OF ORAL ERYTHROMYCIN STEARATE, 500 MG 4 TIMES A DAY, RESULTED IN A PROTHROMBIN TIME OF 64 SECONDS (CONTROL, 11 SECONDS). Non-Human Toxicity Values LD50 Rat oral 9272 mg/kg LD50 Mouse ip 463 mg/kg LD50 Mouse sc 1800 mg/kg LD50 Mouse im 426 mg/kg For more Non-Human Toxicity Values (Complete) data for Erythromycin (6 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Antibiotics, Macrolide; Gastrointestinal Agents; Protein Synthesis Inhibitors MEDICATION (VET): In veterinary medicine, /erythromycin/ is used the treatment of clinical and subclinical mastitis in lactating cows, for the treatment of infectious diseases due to erythromycin-sensitive bacteria (cattle, sheep, swine, poultry) and for the treatment of chronic respiratory diseases due to mycoplasma in poultry. Erythromycin is used as an alternative agent in the treatment of anthrax. Parenteral penicillins generally have been considered the drugs of choice for the treatment of naturally occurring or endemic anthrax caused by susceptible strains of Bacillus anthracis, including clinically apparent GI, inhalational, or meningeal anthrax and anthrax septicemia, although IV ciprofloxacin or IV doxycycline also are recommended. Erythromycin is suggested as an alternative to penicillin G for the treatment of naturally occurring or endemic anthrax in patients hypersensitive to penicillins. ./NOT included in US product label/ Erythromycin is used topically in the treatment of acne vulgaris. Therapy of acne vulgaris must be individualized and frequently modified depending on the types of acne lesions which predominate and the response to therapy. Topical anti-infectives, including erythromycin, are generally effective in the treatment of mild to moderate inflammatory acne. However, use of topical anti-infectives as monotherapy may lead to bacterial resistance; this resistance is associated with decreased clinical efficacy. Topical erythromycin is particularly useful when used with benzoyl peroxide or topical retinoids. Results of clinical studies indicate that combination therapy results in a reduction in total lesion counts of 50 to 70%. /Included in US product label/ For more Therapeutic Uses (Complete) data for Erythromycin (23 total), please visit the HSDB record page. ITS ACTIONS & USES ARE IDENTICAL TO THOSE OF ERYTHROMYCIN. ERYTHROMYCIN MAY BE USEFUL FOR DISSEMINATED GONOCOCCAL DISEASE IN PREGNANT PT WHO IS ALLERGIC TO PENICILLIN... 13 PT...TREATED WITH 500 MG OF ERYTHROMYCIN... STEARATE, GIVEN ORALLY EVERY 6 HR FOR 5 DAYS, SHOWED RAPID CLINICAL & BACTERIOLOGICAL RESPONSES. ANTIBACTERIAL AGENT MEDICATION (VET): ANTIBACTERIAL AGENT Drug Warnings Some commercially available formulations of erythromycin lactobionate powder for injection contain benzyl alcohol as a preservative. Although a causal relationship has not been established, administration of injections preserved with benzyl alcohol has been associated with toxicity in neonates. Toxicity appears to have resulted from administration of large amounts (i.e., about 100-400 mg/kg daily) of benzyl alcohol in these neonates. Although use of drugs preserved with benzyl alcohol should be avoided in neonates whenever possible, the American Academy of Pediatrics states that the presence of small amounts of the preservative in a commercially available injection should not proscribe its use when indicated in neonates. /Erythromycin lactobionate/ In several neonates with infections caused by Ureaplasma urealyticum who received IV administration of erythromycin lactobionate, adverse cardiac effects (e.g., bradycardia, hypotension, cardiac arrest, arrhythmias) requiring cardiopulmonary resuscitation have been reported. Some clinicians state that these adverse effects may depend on serum concentration and/or infusion rate of the drug. It has been suggested that prolonged IV infusion of erythromycin lactobionate (e.g., over 60 minutes) may reduce such adverse cardiac effects. However, it has been suggested that certain individuals may be at increased risk of developing erythromycin-induced adverse cardiac effects and that decreasing the rate of IV infusion may decrease but not eliminate the risk of such effects. Further study is needed to determine the pharmacokinetics and safety of erythromycin lactobionate in neonates. /Erythromycin lactobionate/ Maternal Medication usually Compatible with Breast-Feeding: Erythromycin: Reported Sign or Symptom in Infant or Effect on Lactation: None. /From Table 6/ POTENTIAL ADVERSE EFFECTS ON FETUS: None known. POTENTIAL SIDE EFFECTS ON BREAST-FED INFANT: None known, although theoretically could cause diarrhea in infant. COMMENTS: Crosses placenta in high doses to fetal level 24% of maternal; breast milk may exceed maternal serum concentration. FDA Category: B (B = Studies in laboratory animals have not demonstrated a fetal risk, but there are no controlled studies in pregnant women; or animal studies have shown an adverse effect (other than a decrease in fertility), but controlled studies in pregnant women have not demonstrated a risk to the fetus in the first trimester and there is no evidence of a risk in later trimesters.) /From Table II/ For more Drug Warnings (Complete) data for Erythromycin (17 total), please visit the HSDB record page. ...ERYTHROMYCIN & ITS DERIV SELDOM CAUSE SERIOUS ADVERSE REACTIONS. Pharmacodynamics Macrolides, such as erythromycin, stop bacterial growth by inhibiting protein synthesis and translation, treating bacterial infections. Erythromycin does not exert effects on nucleic acid synthesis. This drug has been shown to be active against most strains of the following microorganisms, effectively treating both in vitro and clinical infections. Despite this, it is important to perform bacterial susceptibility testing before administering this antibiotic, as resistance is a common issue that may affect treatment. **A note on antimicrobial resistance, pseudomembranous colitis, and hepatotoxicity** Many strains of Haemophilus influenzae are resistant to erythromycin alone but are found to be susceptible to erythromycin and sulfonamides used in combination. It is important to note that Staphylococci that are resistant to erythromycin may emerge during erythromycin and/or sulfonamide therapy. Pseudomembranous colitis has been reported with most antibacterial agents, including erythromycin, and may range in severity from mild to life-threatening. Therefore, the physician should consider this diagnosis in patients with diarrhea after the administration of antibacterial agents. Erythromycin can cause hepatic dysfunction, cholestatic jaundice, and abnormal liver transaminases, particularly when erythromycin estolate is administered. |
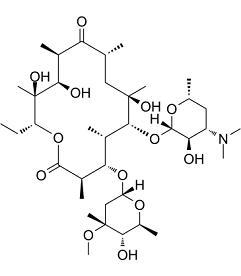
| 分子式 |
C37H67NO13
|
|---|---|
| 分子量 |
733.9268
|
| 精确质量 |
733.461
|
| 元素分析 |
C, 60.55; H, 9.20; N, 1.91; O, 28.34
|
| CAS号 |
114-07-8
|
| 相关CAS号 |
Erythromycin-d6;959119-25-6;Erythromycin-d3;959119-26-7;Erythromycin Ethylsuccinate;1264-62-6;Erythromycin stearate;643-22-1;Erythromycin lactobionate;3847-29-8;Erythromycin (aspartate);30010-41-4;Erythromycin thiocyanate;7704-67-8;Erythromycin A dihydrate;59319-72-1;Erythromycin-13C,d3;2378755-50-9
|
| PubChem CID |
12560
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
818.4±65.0 °C at 760 mmHg
|
| 熔点 |
138-140ºC
|
| 闪点 |
448.8±34.3 °C
|
| 蒸汽压 |
0.0±0.6 mmHg at 25°C
|
| 折射率 |
1.535
|
| 来源 |
Streptomyces erythreHs
|
| LogP |
2.83
|
| tPSA |
193.91
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
14
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
51
|
| 分子复杂度/Complexity |
1180
|
| 定义原子立体中心数目 |
18
|
| SMILES |
O([C@@]1([H])[C@@]([H])([C@]([H])(C([H])([H])[C@@]([H])(C([H])([H])[H])O1)N(C([H])([H])[H])C([H])([H])[H])O[H])[C@@]1([H])[C@@](C([H])([H])[H])(C([H])([H])[C@@]([H])(C([H])([H])[H])C([C@]([H])(C([H])([H])[H])[C@]([H])([C@@](C([H])([H])[H])([C@@]([H])(C([H])([H])C([H])([H])[H])OC([C@]([H])(C([H])([H])[H])[C@]([H])([C@]1([H])C([H])([H])[H])O[C@@]1([H])C([H])([H])[C@](C([H])([H])[H])([C@]([H])([C@]([H])(C([H])([H])[H])O1)O[H])OC([H])([H])[H])=O)O[H])O[H])=O)O[H]
|
| InChi Key |
ULGZDMOVFRHVEP-RWJQBGPGSA-N
|
| InChi Code |
InChI=1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
|
| 化学名 |
(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-6-(((2S,3R,4S,6R)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-14-ethyl-7,12,13-trihydroxy-4-(((2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-3,5,7,9,11,13-hexamethyloxacyclotetradecane-2,10-dione
|
| 别名 |
Emycin; HSDB 3074; HSDB-3074; HSDB3074; Eryc-125; Eryc-250; Erythromycin
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 100 mg/mL (136.25 mM)
Ethanol : ~100 mg/mL H2O : 1 mg/mL (1.36 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (2.83 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (2.83 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (2.83 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10% DMSO+40% PEG300+5% Tween-80+45% Saline: ≥ 2.08 mg/mL (2.83 mM) 配方 5 中的溶解度: 5 mg/mL (6.81 mM) in 0.5% CMC-Na 0.1% Tween-80 (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3625 mL | 6.8126 mL | 13.6253 mL | |
| 5 mM | 0.2725 mL | 1.3625 mL | 2.7251 mL | |
| 10 mM | 0.1363 mL | 0.6813 mL | 1.3625 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Effect of Erythromycin on the Absorption, Metabolism and Elimination of CHF6001 in Healthy Volunteers
CTID: NCT06395610
Phase: Phase 1 Status: Completed
Date: 2024-07-03