| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| Other Sizes |

| 体内研究 (In Vivo) |
盐酸乙胺丁醇可用于动物模型制作高尿酸模型。
|
|---|---|
| 参考文献 | |
| 其他信息 |
Ethambutol hydrochloride is an antibacterial prescription medicine approved by the U.S. Food and Drug Administration (FDA) for the treatment of active tuberculosis (TB) of the lungs. (Active TB is also called TB disease.)
TB can be an opportunistic infection (OI) of HIV. Ethambutol Hydrochloride is the hydrochloride salt form of ethambutol, an ethylenediamine derivative with antibacterial activity, specifically effective against mycobacteria. Although the exact mechanism of action of ethambutol hydrochloride is unknown, ethambutol hydrochloride inhibits the transfer of mycolic acids into the cell wall of bacteria, which impedes bacterial cell growth. This agent may also interfere with RNA synthesis or inhibit other cell metabolism, thereby preventing cell multiplication and causing cell death. An antitubercular agent that inhibits the transfer of mycolic acids into the cell wall of the tubercle bacillus. It may also inhibit the synthesis of spermidine in mycobacteria. The action is usually bactericidal, and the drug can penetrate human cell membranes to exert its lethal effect. (From Smith and Reynard, Textbook of Pharmacology, 1992, p863) |
| 精确质量 |
276.137
|
|---|---|
| CAS号 |
1070-11-7
|
| 相关CAS号 |
Ethambutol;74-55-5
|
| PubChem CID |
14051
|
| 外观&性状 |
White to off-white solid powder
|
| 沸点 |
345.3ºC at 760 mmHg
|
| 熔点 |
198-200°C
|
| 闪点 |
113.7ºC
|
| 蒸汽压 |
3.35E-07mmHg at 25°C
|
| LogP |
2.093
|
| tPSA |
64.52
|
| 氢键供体(HBD)数目 |
6
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
16
|
| 分子复杂度/Complexity |
109
|
| 定义原子立体中心数目 |
2
|
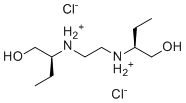
| SMILES |
CC[C@@H](CO)NCCN[C@@H](CC)CO.Cl.Cl
|
| InChi Key |
AUAHHJJRFHRVPV-BZDVOYDHSA-N
|
| InChi Code |
InChI=1S/C10H24N2O2.2ClH/c1-3-9(7-13)11-5-6-12-10(4-2)8-14;;/h9-14H,3-8H2,1-2H3;2*1H/t9-,10-;;/m0../s1
|
| 化学名 |
(2S)-2-[2-[[(2S)-1-hydroxybutan-2-yl]amino]ethylamino]butan-1-ol;dihydrochloride
|
| 别名 |
Ethambutol Hydrochloride Ethambutol HCL 4878
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~360.71 mM)
H2O : ≥ 50 mg/mL (~180.36 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 100 mg/mL (360.71 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00002343 | COMPLETED | Drug: Ethambutol hydrochloride Drug: Rifabutin |
HIV Infections Mycobacterium Avium-Intracellulare Infection |
Pharmacia | Phase 4 | |
| NCT01048697 | COMPLETEDWITH RESULTS | Drug: Ethambutol | Obesity Tuberculosis |
Texas Tech University Health Sciences Center | 2010-01 | Phase 4 |
| NCT01994460 | UNKNOWN STATUS | Drug: Linezolid Drug: Ethambutol |
Pulmonary Tuberculosis Without Resistance to Rifampicin | Seoul National University Hospital | 2014-01 | Phase 2 |
| NCT05966688 | COMPLETED | Drug: SPR720 Drug: Azithromycin Drug: Ethambutol |
Healthy Volunteers | Spero Therapeutics | 2023-08-04 | Phase 1 |
| NCT04972903 | UNKNOWN STATUS | Pulmonary Tuberculosis |
Institut National de la Santé Et de la Recherche Médicale, France |
2021-08 |