| 规格 | 价格 | ||
|---|---|---|---|
| 500mg | |||
| 1g | |||
| Other Sizes |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Oral ethambutol is approximately 75-80% orally bioavailable. A 25 mg/kg oral dose of ethambutol reaches a Cmax of 2-5 µg/mL, with a Tmax of 2-4 hours. In a separate study, the AUC0-8 varied from 6.3 ± 5.5 h\*mg/L to 10.8 ± 7.6 h\*mg/L depending on CYP1A2 genetic polymorphisms. Ethambutol is 50% eliminated in the urine as the unmetabolized parent compound and 8-15% as inactive metabolites. 20-22% of a dose is eliminated unchanged in the feces. Patients coinfected with tuberculosis and HIV have an estimated ethambutol volume of distribution of 76.2 L. Patients coinfected with tuberculosis and HIV have an estimated ethambutol oral clearance of 77.4 L/h. Approximately 75-80% of an oral dose of ethambutol hydrochloride is rapidly absorbed from the GI tract. Absorption is not substantially affected when the drug is administered with food. Following a single oral ethambutol hydrochloride dose of 25 mg/kg, peak serum ethambutol concentrations of 2-5 mcg/mL are attained within 2-4 hours; serum concentrations of the drug are undetectable 24 hours after the dose. There is no evidence that accumulation of the drug occurs when ethambutol doses of 25 mg/kg are given once daily in patients with normal renal function. Serum concentrations of the drug are higher and accumulation may occur when ethambutol is used in patients with impaired renal function. Ethambutol is widely distributed into most body tissues and fluids. Highest concentrations of the drug are found in erythrocytes, kidneys, lungs, and saliva; lower drug concentrations are found in ascitic fluid, pleural fluid, brain, and CSF. Peak intracellular concentrations of ethambutol in erythrocytes are about twice peak plasma concentrations and maintain this ratio for at least 24 hours after a single oral dose. In patients with meningitis, administration of an oral ethambutol hydrochloride dose of 25 mg/kg has produced peak CSF concentrations of the drug ranging from 0.15-2.0 ug/mL. /Ethambutol/ does not penetrate intact meninges, but 10 to 50% may penetrate the meninges of patients with tuberculous meningitis. Volume of distribution is 1.6 liters per kg For more Absorption, Distribution and Excretion (Complete) data for ETHAMBUTOL (9 total), please visit the HSDB record page. Metabolism / Metabolites Ethambutol is mainly oxidized by an aldehyde dehydrogenase to an aldehyde metabolite, followed by conversion to the dicarboxylic acid 2,2'-(ethylinediimino)di-butyric acid. ... Up to 15% is excreted in the form of two metabolites, an aldehyde and a dicarboxylic acid derivative. Ethambutol is partially inactivated in the liver by oxidation to an aldehyde intermediate, 2,2?-(ethylenediimino)-di-butyraldehyde, which is converted to the decarboxylic acid derivative, 2,2?-(ethylenediimino)-di-butyric acid. Biological Half-Life Ethambutol has a half life of 3.3 hours in patients with normal renal function. In patients with renal failure, the half life could be 7 hours or longer. The plasma half-life of ethambutol is approximately 3.3 hours in patients with normal renal function. The half-life is prolonged in patients with impaired renal or hepatic function. In patients with renal failure, the half-life may be 7 hours or longer. Six normal adult volunteers were administered 15 mg/kg of ethambutol (EMB) by a constant-rate 1-hr infusion. Plasma and urine samples were collected up to 24 and 72 hr, respectively. ... Subsequent postinfusion EMB levels exhibited multiphasic decay. In the 12-hr period following infusion, EMB levels showed biexponential decay. However, 24-hr plasma levels in all subjects were observed to be higher than those predicted using a two-compartment body model. The alpha phase in these subjects had a mean half-life of 8.6 min while the half-life of the beta phase ranged from 2.5 to 3.6 hr (mean 3.1). The half-life of the gamma phase estimated from plasma data points between 12 and 24 hr averaged 1.2 +/- 3.6 hr. A terminal gamma t1/2 of 15.4 +/- 1.7 hr was calculated from 12-72 hr urine data. ... Plasma EMB clearance ranged from 7.47 to 8.87 mL/min/kg (mean 8.57). ... |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION: Ethambutol is used to treat tuberculosis. Ethambutol is an odorless crystalline hygroscopic powder. It is soluble in water, alcohol, chloroform, methyl alcohol, and very slightly soluble in ether. HUMAN EXPOSURE: Summary: Main risks and target organs: During chronic treatment ethambutol may produce visual and neurological disturbances, allergic reactions, gastrointestinal symptoms, psychiatric symptoms and transient impairment of liver function. This last event has a very low incidence. Increased serum uric acid levels and acute gouty arthritis have been reported. Summary of clinical effects: Acute overdosage may cause gastrointestinal symptoms, hallucinations and optic neuritis. Acute overdosage symptoms include nausea, abdominal pain, fever, mental confusion, visual hallucinations, and optic neuropathy (retrobulbar neuritis) with doses over 10 g. The effects of overdosage are not well established. During chronic treatment the following have been reported: Visual disturbances: Ethambutol may produce a reduction of visual acuity which appear to be due to optic neuritis. Central scotoma and green-red colour blindness may also occur. Allergic reactions: Rash, anaphylactoid reactions, dermatitis and pruritus. Gastrointestinal symptoms: Abdominal pain, anorexia, nausea, vomiting. Neurological disturbances and psychiatric symptoms: Headache, peripheral neuritis, dizziness, mental confusion, disorientation and hallucinations. Other side effects: Jaundice, transient impairment of liver function, fever, increase of serum uric acid levels, joint pain, acute gouty arthritis, malaise. Ethambutol may diffuse into milk. Ethambutol is a synthetic oral antibiotic derivative of ethylenediamine. Contraindications: Ethambutol hydrochloride is contraindicated in patients who are known to be hypersensitive to this drug. Renal impairment, old age and optic neuritis are relative contraindications. Routes of entry: Oral: Ethambutol is only available for oral use. Absorption by route of exposure: About 80% of an oral dose of ethambutol is absorbed from the gastro-intestinal tract, and the remainder appears in the feces unchanged. Absorption is not significantly impaired by food. Distribution by route of exposure: Ethambutol diffuses readily into red blood cells and into the cerebrospinal fluid when the meninges are inflamed. The concentration in erythrocytes at steady state is approximately twice the plasma concentration. It has been reported to cross the placenta and is excreted in breast milk. Biological half-life by route of exposure: The serum half-life in therapeutic doses is 3 hours, increasing in renal failure, as 80% is excreted renally. Metabolism: The main path of metabolism appears to be an initial oxidation of the alcohol to an aldehydic intermediate, followed by conversion to a dicarboxylic acid. Elimination by route of exposure: During the 24 hour period following oral administration of ethambutol, approximately 50% of the initial dose is excreted unchanged in the urine, while an additional 8% to 15% appears in the form of metabolites. From 20 to 22% of the initial dose is excreted in the feces as unchanged drug. Mode of action: Toxicodynamics: The underlying cause of visual alterations appears to be a disturbance of metabolism due to depletion of copper and zinc which serve as prosthetic groups for many enzymes. The eye normally contains a considerable store of zinc. Much of the zinc is in the pigmented cells of the outer zone of the retina, where it serves as a metal prosthetic group for retinol (alcohol) dehydrogenase. Pharmacodynamics: Ethambutol is an oral chemotherapeutic agent which is specifically effective against actively growing microorganisms of the genus Mycobacterium, including M. tuberculosis. Ethambutol is bacteriostatic and appears to inhibit the synthesis of one or more metabolites, thus causing impairment of cell metabolism, arrest of multiplication, and cell death. No cross resistance with other available antimycobacterial agents has been demonstrated. Ethambutol has been shown to be effective against strains of mycobacterium tuberculosis but does not seem to be active against fungi, viruses, or other bacteria. Ethambutol is also active against some atypical mycobacteria including M. kansasii. Primary resistance to ethambutol is uncommon in developed countries but resistant strains of M. tuberculosis are readily produced if the drug is used alone. Human data: Adults: Subclinical impairment of colour discrimination was reported to be relatively common in patients receiving ethambutol daily as part of antituberculous chemotherapy when compared with 50 patients receiving other antituberculous agents. Peripheral neuropathy has been reported in tubercular patients who had received ethambutol among other drugs. Interactions: Results of a crossover study involving 13 tuberculous patients suggest that concomitant administration of aluminium hydroxide may delay and reduce absorption of ethambutol in some patients. Untoward effects may be enhanced when ethambutol is combined with isoniazid or rifampicin. Main adverse effects: Ethambutol may produce decreased visual acuity which appear to be due to optic neuritis and to be related to dose and duration of treatment. The effects are generally reversible when administration of the drug is discontinued promptly. Ethambutol may produce constriction of visual field, central and peripheral scotoma, and green-red color blindness which may be associated with retrobulbar neuritis. Renal clearance of urate may be reduced in about 50% of patients receiving ethambutol and acute gout has been precipitated in patients with gout or impaired renal function. Cholestatic jaundice has been reported. ANIMAL/PLANT STUDIES: Relevant animal data: Toxicological studies in dogs on high prolonged doses, produced evidence of myocardial damage and failure, and depigmentation of the tapetum lucidum of the eyes, the significance of which is not known. Degenerative changes in the central nervous system, apparently not dose-related, have also been noted in dogs receiving ethambutol hydrochloride over a prolonged period. In the rhesus monkey, neurological signs appeared after treatment with high doses given daily over a period of several months. These correlated with specific serum levels of ethambutol hydrochloride and with definite neuro-anatomical changes in the central nervous system. Focal interstitial carditis was also noted in monkeys which received ethambutol hydrochloride in high doses for a prolonged period. Interactions Patient administered digitoxin (0.1 mg/day orally) while taking ethambutol (20-25 mg/kg/day) had markedly decreased serum levels of digitoxin compared to controls (16.6 versus 35 ng/mL). Binding of digitoxin by blood proteins was same in both groups. Its metabolism was probably increased. Concurrent administration of ethambutol with other neurotoxic medications may increase the potential for neurotoxicity, such as optic and peripheral neuritis. Aluminum salts may delay and reduce the absorption of ethambutol. The effect of repeated administration of rifabutin on the pharmacokinetics and metabolism of ethambutol was evaluated in ten healthy volunteers. The subjects received a single oral administration of 1200 mg ethambutol on days 1 and 10 and a single daily oral dose of 300 mg rifabutin from days 3 to 9. No statistically significant difference was found in plasma pharmacokinetics (C(max), t(max), AUC, half-life and MRT) and in the renal clearance, whereas a significant decrease in the amount of unchanged ethambutol excreted in urine was observed. The decrease observed in ethambutol urinary excretion may be accounted for by taking into consideration the variability of the urinary excretion of ethambutol reported in the literature. However, a slight, likely not clinically relevant, induction or activation of kidney alcohol and/or aldehyde dehydrogenase isoenzymes by rifabutin cannot be ruled out at present. Evidence exists in the present study for autoinduction of rifabutin metabolism; this is shown by the lower plasma concentrations obtained 24 hr after the seventh dose as compared to the theoretical concentrations. Non-Human Toxicity Values LD50 Mouse oral 2800 mg/kg /Mixture with isoniazid methane sulfonate/ LD50 Mouse ip 2210 mg/kg /Mixture with isoniazid methane sulfonate/ |
| 参考文献 |
Safi H, Lingaraju S, Amin A, Kim S, Jones M, Holmes M, McNeil M, Peterson SN, Chatterjee D, Fleischmann R, Alland D. Evolution of high-level ethambutol-resistant tuberculosis through interacting mutations in decaprenylphosphoryl-β-D-arabinose biosynthetic and utilization pathway genes. Nat Genet. 2013 Oct;45(10):1190-7. doi: 10.1038/ng.2743. Epub 2013 Sep 1. PubMed PMID: 23995136.
|
| 其他信息 |
Therapeutic Uses
Antitubercular Agents Ethambutol is indicated in combination with other antituberculosis medications in the treatment of all forms of tuberculosis, including tuberculous meningitis, caused by Mycobacterium tuberculosis. /Included in US product labeling/ Ethambutol is used in the treatment of atypical mycobacterial infections, such as Mycobacterium avium complex (MAC). /NOT included in US product labeling/ Drug Warnings Appropriate studies on the relationship of age to the effects of ethambutol have not been performed in children up to 13 years of age. Ethambutol is generally not recommended in children whose visual acuity cannot be monitored (younger than 6 years of age). However, ethambutol should be considered for all children with organisms resistant to other medications, and in whom susceptibility to ethambutol has been demonstrated or is likely. The most important adverse effect of ethambutol is optic neuritis with decreases in visual acuity, constriction of visual fields, central and peripheral scotomas, and loss of red-green color discrimination. The extent of ocular toxicity appears to be related to the dose and duration of ethambutol therapy. However, such toxicity also has been reported rarely after only a few days of therapy with the drug, and may represent an idiosyncratic reaction. Other adverse effects of ethambutol include dermatitis, pruritus, headache, malaise, dizziness, fever, mental confusion, disorientation, possible hallucinations, joint pain, and rarely anaphylactoid reactions. GI upset, abdominal pain, nausea, vomiting, and anorexia have also occurred occasionally with ethambutol. Peripheral neuritis, with numbness and tingling of the extremities, has been reported infrequently. Increased serum uric acid concentrations and precipitation of acute gout have occurred occasionally in patients receiving ethambutol and are probably the result of decreased renal clearance of urate. Transient impairment of liver function, as indicated by abnormal liver function test results, has also occurred. Cholestatic jaundice, which appeared to be caused by ethambutol, has been reported in at least one patient who received the drug both alone and in conjunction with streptomycin. Visual testing should be performed prior to initiating ethambutol therapy and then periodically during therapy with the drug. Testing should be done monthly in patients receiving more than 15 mg/kg daily. Examinations should include ophthalmoscopy, finger perimetry, and testing of color discrimination. Patients developing adverse ocular effects during ethambutol therapy may show subjective visual symptoms either before or simultaneously with decreases in visual acuity. All patients receiving the drug should be questioned periodically about blurred vision and other subjective visual symptoms and should be instructed to report to their physicians any such changes as soon as they are noticed. If substantial changes in visual acuity occur, ethambutol should be discontinued immediately. For more Drug Warnings (Complete) data for ETHAMBUTOL (10 total), please visit the HSDB record page. Pharmacodynamics Ethambutol is indicated in combination with other anti-tuberculosis drugs in the treatment of pulmonary tuberculosis. It has a long duration of action as it is administered daily, and a moderate therapeutic window. Patients should be counselled regarding the risk of optic neuritis and hepatic toxicity. |
| 分子式 |
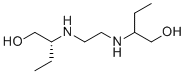
C10H24N2O2
|
|---|---|
| 精确质量 |
204.183
|
| CAS号 |
74-55-5
|
| 相关CAS号 |
Ethambutol dihydrochloride;1070-11-7;Ethambutol-d4;1129526-19-7;Ethambutol-d10;1129526-24-4;Ethambutol-d8;1129526-23-3
|
| PubChem CID |
14052
|
| 外观&性状 |
Crystals
WHITE, CRYSTALLINE POWDER |
| 密度 |
1.0±0.1 g/cm3
|
| 沸点 |
345.3±22.0 °C at 760 mmHg
|
| 熔点 |
199 - 204ºC
|
| 闪点 |
113.7±12.9 °C
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
| 折射率 |
1.478
|
| LogP |
-0.05
|
| tPSA |
64.52
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
14
|
| 分子复杂度/Complexity |
109
|
| 定义原子立体中心数目 |
2
|
| SMILES |
CC[C@H](NCCN[C@@H](CC)CO)CO
|
| InChi Key |
AEUTYOVWOVBAKS-YHMJZVADSA-N
|
| InChi Code |
InChI=1S/C10H24N2O2/c1-3-9(7-13)11-5-6-12-10(4-2)8-14/h9-14H,3-8H2,1-2H3/t9-,10?/m1/s1
|
| 化学名 |
1-Butanol, 2,2'-(1,2-ethanediyldiimino)bis-, (R)-
|
| 别名 |
Etambutol Aethambutolum Diambutol Purderal Tibutol
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。