| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg | |||
| 500mg | |||
| Other Sizes |
| 靶点 |
Selective and potent inhibitor of cholesteryl ester transfer protein (CETP) with the following inhibitory parameters:
- IC50 = 1.6 nM (recombinant human CETP), Ki = 0.8 nM (recombinant human CETP); - No significant inhibition of other lipid-related enzymes, including monoacylglycerol lipase (MAGL) and lecithin-cholesterol acyltransferase (LCAT) (inhibition rate <2% at 10 μM) [1] |
|---|---|
| 体外研究 (In Vitro) |
新型 CETP 抑制剂 evetrapib 基于苯并氮杂卓。缓冲液 CETP 测定中药物的绝对效力为 5.5 nM。对于人血浆CETP测定,来自人血浆的重组蛋白或CETP的CETP浓度约为2 μg/mL (25 nM),36 nM IC50值证实依泽帕尼是强CETP抑制剂。据一些报道,evacetrapib 比 dalecapib 更有效[1]。
CETP活性抑制与脂质调节: - 在重组人CETP实验中,依伐曲匹布(Evacetrapib) (0.1~100 nM)以浓度依赖性方式抑制CETP介导的胆固醇酯(CE)从HDL向LDL转移: - 0.1 nM浓度抑制18%的CE转移; - 1 nM浓度抑制65%的CE转移; - 10 nM浓度抑制90%的CE转移; - 100 nM浓度抑制率>95%。 - 在人血浆孵育体系中,100 nM 依伐曲匹布(Evacetrapib) 使高密度脂蛋白胆固醇(HDL-C)水平升高35%,低密度脂蛋白胆固醇(LDL-C)水平降低20%(酶法检测)[1] - 不诱导醛固酮合成: - 在原代人肾上腺皮质细胞中,依伐曲匹布(Evacetrapib) (1~100 μM)未显著改变醛固酮分泌:醛固酮水平维持在溶剂对照组的5%范围内。相比之下,参考CETP抑制剂托彻普(10 μM)在相同条件下使醛固酮分泌升高40%[1] |
| 体内研究 (In Vivo) |
在表达人 CETP 和 apoAI 的双转基因小鼠中,口服给药后 8 小时,Evacetrapib 显示 HDL 胆固醇显着增加,且离体 CETP 抑制 ED50 低于 5 mg/kg。至关重要的是,当给予高剂量依折麦布暴露倍数的大鼠与阳性对照托塞曲匹(torcetrapib)进行比较时,血压没有升高。口服时,剂量为30 mg/kg的依折麦布在给药后4、8和24小时分别抑制CETP活性98.4%、98.6%和18.4%。口服给药后 8 小时,当依折麦布剂量为 30 mg/kg 时,HDL-C 增加了 129.7%[1]。
小鼠体内脂质调节与安全性: - 对雄性C57BL/6小鼠,口服依伐曲匹布(Evacetrapib) (1 mg/kg/天、3 mg/kg/天、10 mg/kg/天)14天: - HDL-C水平较溶剂组分别升高30%(1 mg/kg)、55%(3 mg/kg)、80%(10 mg/kg); - LDL-C水平较溶剂组分别降低10%(1 mg/kg)、18%(3 mg/kg)、25%(10 mg/kg); - 收缩压/舒张压及血浆醛固酮水平与溶剂组无显著差异(血压变化<1 mmHg,醛固酮变化<8%)[1] - 恒河猴体内脂质调节与安全性: - 对雄性恒河猴,口服依伐曲匹布(Evacetrapib) (3 mg/kg/天、10 mg/kg/天)21天: - HDL-C水平较溶剂组分别升高50%(3 mg/kg)、75%(10 mg/kg); - LDL-C水平较溶剂组分别降低22%(3 mg/kg)、30%(10 mg/kg); - 血压(收缩压/舒张压变化<2 mmHg)、血浆醛固酮及血清肝肾功能标志物(ALT、AST、BUN、肌酐)均无显著变化[1] |
| 酶活实验 |
重组人CETP活性检测:
反应体系(200 μL)包含50 mM Tris-HCl(pH 7.4)、1 mM EDTA、0.1%牛血清白蛋白(BSA)、50 μg/mL [14C]-胆固醇油酸酯标记人HDL([14C]-CE-HDL)、50 μg/mL人LDL及依伐曲匹布(Evacetrapib) (0.01~100 nM)。混合物在37°C孵育4小时,以允许CETP介导CE从HDL向LDL转移。加入500 μL冰浴硫酸葡聚糖-MgCl2溶液(沉淀LDL)终止反应,3000×g、4°C离心15分钟后,收集上清液(含未转移的[14C]-CE-HDL),通过液体闪烁计数器检测其放射性。药物处理组与溶剂组的放射性差异用于计算CE转移抑制率,使用GraphPad Prism软件拟合浓度-抑制曲线得到IC50,通过双倒数作图法(Lineweaver-Burk plot,改变[14C]-CE-HDL浓度:10~100 μg/mL)计算Ki值[1] - 酶选择性检测: 采用相同反应缓冲液,检测10 μM 依伐曲匹布(Evacetrapib) 对MAGL和LCAT的抑制作用以评估选择性: - MAGL检测:底物为[3H]-2-花生四烯酰甘油([3H]-2-AG,20 nM),37°C孵育30分钟,萃取水解产物([3H]-花生四烯酸)并通过液体闪烁计数定量; - LCAT检测:底物为[14C]-胆固醇标记人HDL([14C]-Chol-HDL,50 μg/mL),37°C孵育2小时,通过薄层色谱分离生成的[14C]-CE并经放射性扫描定量。两种酶的抑制率均<2%,证实其对CETP的高选择性[1] |
| 细胞实验 |
原代人肾上腺皮质细胞醛固酮分泌实验:
1. 细胞分离与培养:从正常肾上腺组织中分离原代人肾上腺皮质细胞,以1×105细胞/孔接种于24孔板,在含10%胎牛血清(FBS)、100 U/mL青霉素和100 μg/mL链霉素的DMEM/F12培养基中,37°C、5% CO2培养48小时至融合度达80%[1] 2. 药物处理:更换为含依伐曲匹布(Evacetrapib) (1 μM、10 μM、100 μM)或托彻普(10 μM,阳性对照)的无血清DMEM/F12培养基;溶剂对照组加入含0.1% DMSO的培养基,细胞继续培养24小时[1] 3. 醛固酮检测:收集培养上清液,1200×g离心5分钟去除细胞碎片,使用商品化酶联免疫吸附测定(ELISA)试剂盒检测醛固酮浓度。酶标仪读取450 nm处吸光度,参照标准曲线计算醛固酮水平[1] |
| 动物实验 |
Dissolved in 10% acacia; 30 mg/kg; Oral gavage
Human ApoAI and CETP double transgenic mice C57BL/6 mouse study: 1. Animals and grouping: Male C57BL/6 mice (8–10 weeks old, 20–25 g) were randomly divided into 4 groups (n=8 per group): vehicle control (0.5% carboxymethyl cellulose sodium, CMC-Na), Evacetrapib 1 mg/kg, 3 mg/kg, and 10 mg/kg [1] 2. Drug preparation: Evacetrapib was dissolved in 0.5% CMC-Na to prepare homogeneous suspensions (sonicated for 5 minutes to ensure solubility) [1] 3. Administration: Mice received daily oral gavage at a volume of 10 mL/kg for 14 days. The vehicle group received the same volume of 0.5% CMC-Na [1] 4. Sample collection and detection: - Blood pressure: Measured weekly using a non-invasive tail-cuff blood pressure system (before gavage, mice were acclimated for 5 minutes at 37°C). - Blood lipids: On day 14, mice were fasted for 6 hours, then blood was collected via orbital sinus. Serum HDL-C and LDL-C were measured by enzymatic kits. - Plasma aldosterone: Quantified by ELISA using plasma separated from blood samples [1] - Rhesus monkey study: 1. Animals and grouping: Male rhesus monkeys (5–7 years old, 5–7 kg) were randomly divided into 3 groups (n=4 per group): vehicle control (0.5% CMC-Na), Evacetrapib 3 mg/kg, and 10 mg/kg [1] 2. Administration: Daily oral gavage for 21 days (volume: 5 mL/kg). The vehicle group received 0.5% CMC-Na [1] 3. Sample collection and detection: - Blood pressure: Measured weekly using a non-invasive arm-cuff system (monkeys were sedated with ketamine before measurement). - Blood lipids: Weekly fasting blood samples (12-hour fast) were collected from the femoral vein, and serum HDL-C/LDL-C were measured. - Safety markers: On day 21, serum ALT, AST, BUN, creatinine (liver/kidney function) and plasma aldosterone were measured [1] |
| 药代性质 (ADME/PK) |
Plasma protein binding: Evacetrapib showed high plasma protein binding (>99%) in human, mouse, and rhesus monkey plasma, determined by equilibrium dialysis (dialysis buffer: 50 mM Tris-HCl, pH 7.4; incubation time: 4 hours at 37°C) [1]
- Oral bioavailability: In rhesus monkeys, oral administration of Evacetrapib (10 mg/kg) resulted in an oral bioavailability (F) of 68%, with a time to reach maximum concentration (Tmax) of 4 hours and a maximum plasma concentration (Cmax) of 320 ng/mL [1] - Half-life (t1/2): In rhesus monkeys, the elimination half-life of Evacetrapib was 18 hours (after oral administration of 10 mg/kg) [1] - Tissue distribution: In mice treated with 10 mg/kg oral Evacetrapib (day 14), the highest tissue concentrations were observed in the liver (12 μg/g) and adipose tissue (8.5 μg/g), while brain penetration was low (0.1 μg/g) [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Acute toxicity: Single oral administration of Evacetrapib up to 300 mg/kg in mice caused no mortality or obvious toxic signs (e.g., lethargy, weight loss, ataxia) within 72 hours [1]
- Liver/kidney safety: In mice (10 mg/kg/day for 14 days) and rhesus monkeys (10 mg/kg/day for 21 days), serum levels of ALT, AST, BUN, and creatinine were not significantly different from the vehicle control group (all within normal physiological ranges) [1] - Blood pressure and aldosterone safety: Unlike torcetrapib, Evacetrapib did not induce dose-dependent blood pressure elevation or aldosterone synthesis in mice or monkeys: - In mice (10 mg/kg/day), systolic/diastolic blood pressure changed by <1 mmHg, and plasma aldosterone changed by <8%. - In monkeys (10 mg/kg/day), systolic/diastolic blood pressure changed by <2 mmHg, and plasma aldosterone remained unchanged [1] |
| 参考文献 | |
| 其他信息 |
Evacetrapib is a benzazepine.
Evacetrapib has been used in trials studying the basic science and treatment of Dyslipidemia, Hyperlipidemia, Hypercholesterolemia, Hepatic Insufficiency, and Cardiovascular Disease. Drug Indication Treatment of hypercholesterolaemia, Treatment of hypo-high-density-lipoprotein-cholesterolaemia Evacetrapib (LY2484595) is a synthetic, potent, and selective CETP inhibitor developed for the treatment of atherosclerotic cardiovascular disease (ASCVD), with a core advantage of avoiding the safety issues (blood pressure elevation, aldosterone induction) associated with earlier CETP inhibitors such as torcetrapib [1] - Its mechanism of action involves inhibiting CETP-mediated transfer of cholesteryl esters from HDL (the "protective cholesterol") to LDL/VLDL (the "atherogenic cholesterol"), thereby increasing HDL-C levels and promoting reverse cholesterol transport (RCT)—a process that clears excess cholesterol from peripheral tissues to the liver for excretion [1] - Preclinical studies in mice and rhesus monkeys confirm that Evacetrapib exhibits dose-dependent lipid-regulating effects (elevating HDL-C, reducing LDL-C) while maintaining safety in terms of blood pressure, aldosterone balance, and liver/kidney function, supporting its potential for clinical development [1] |
| 分子式 |
C31H36F6N6O2
|
|
|---|---|---|
| 分子量 |
638.65
|
|
| 精确质量 |
638.28
|
|
| CAS号 |
1186486-62-3
|
|
| 相关CAS号 |
|
|
| PubChem CID |
49836058
|
|
| 外观&性状 |
White to light yellow solid powder
|
|
| 密度 |
1.4±0.1 g/cm3
|
|
| 沸点 |
680.1±65.0 °C at 760 mmHg
|
|
| 闪点 |
365.1±34.3 °C
|
|
| 蒸汽压 |
0.0±2.2 mmHg at 25°C
|
|
| 折射率 |
1.598
|
|
| LogP |
6.22
|
|
| tPSA |
87.38
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
13
|
|
| 可旋转键数目(RBC) |
7
|
|
| 重原子数目 |
45
|
|
| 分子复杂度/Complexity |
973
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
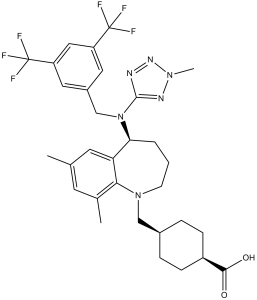
CC1=CC(=C2C(=C1)[C@H](CCCN2CC3CCC(CC3)C(=O)O)N(CC4=CC(=CC(=C4)C(F)(F)F)C(F)(F)F)C5=NN(N=N5)C)C
|
|
| InChi Key |
IHIUGIVXARLYHP-UXNJHFGPSA-N
|
|
| InChi Code |
InChI=1S/C31H36F6N6O2/c1-18-11-19(2)27-25(12-18)26(5-4-10-42(27)16-20-6-8-22(9-7-20)28(44)45)43(29-38-40-41(3)39-29)17-21-13-23(30(32,33)34)15-24(14-21)31(35,36)37/h11-15,20,22,26H,4-10,16-17H2,1-3H3,(H,44,45)/t20?,22?,26-/m0/s1
|
|
| 化学名 |
4-[[(5S)-5-[[3,5-bis(trifluoromethyl)phenyl]methyl-(2-methyltetrazol-5-yl)amino]-7,9-dimethyl-2,3,4,5-tetrahydro-1-benzazepin-1-yl]methyl]cyclohexane-1-carboxylic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (3.91 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (3.91 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 15% Captisol: 30 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5658 mL | 7.8290 mL | 15.6580 mL | |
| 5 mM | 0.3132 mL | 1.5658 mL | 3.1316 mL | |
| 10 mM | 0.1566 mL | 0.7829 mL | 1.5658 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02226653 | Completed Has Results | Drug: Evacetrapib | Healthy Volunteers | Eli Lilly and Company | September 2014 | Phase 1 |
| NCT01903434 | Completed Has Results | Drug: Evacetrapib | Healthy Volunteers | Eli Lilly and Company | July 2013 | Phase 1 |
| NCT01825889 | Completed Has Results | Drug: Evacetrapib | Cardiovascular Disease | Eli Lilly and Company | April 2013 | Phase 1 |
| NCT02497391 | Completed Has Results | Drug: Evacetrapib | Healthy | Eli Lilly and Company | July 2015 | Phase 1 |