| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg | |||
| Other Sizes |
| 靶点 |
SIRT1
|
|---|---|
| 体外研究 (In Vitro) |
(S)-Selisistat 是一种 SIRT1 酶活性抑制剂(IC50,98 nM),是通过使用细菌中表达的人 SIRT1 进行高通量筛选发现的。 (S)-Selisistat 以浓度依赖性方式抑制 SIRT1,其 IC50 为 38 nM,与细菌中表达的 SIRT1 的活性相当。 (S)-Selisistat 在浓度高达 100 μM 时不会抑制 I/II 类 HDAC 活性,但对 SIRT2 (IC50, 19.6 μM) 或 SIRT3 (IC50, 48.7 μM) 的效果明显较差 [1]。 (S)-Selisistat 抑制 SIRT1 活性,而 SIRT1 mRNA 和蛋白质水平不受影响 [2]。
|
| 体内研究 (In Vivo) |
在 ob/ob 脓毒症小鼠中,(S)-Selisistat 消除了白藜芦醇 (RSV) 诱导的微血管炎症的缓解作用。最后,与脓毒症+媒介物组相比,脓毒症+RSV组ob/ob小鼠的7天存活率相当高[3]。
|
| 酶活实验 |
I类和II类HDAC荧光分析。[4]
在上述荧光分析中,使用含有I类和II类HDAC的HeLa细胞提取物和代表赖氨酸16乙酰化组蛋白H4残基12−16的H4-K16(Ac)底物,测量了I类和Ⅱ类HDAC脱乙酰酶活性。 烟酰胺释放试验。[4] 使用代表赖氨酸382乙酰化残基368−386的p53肽底物在非荧光测定中测量SIRT1的活性。如前所述,该测定测量了[14C]烟酰胺从[羰基-14C]-NAD中的释放。 在添加浓度为52μM的未标记烟酰胺的情况下,使用上述测定法测量烟酰胺交换。添加的烟酰胺通过酶催化交换促进[14C]烟酰胺从标记的NAD中释放。在NAD释放[14C]烟酰胺后,未标记的烟酰胺与酶结合并转化为未标记的NAD 如上所述,在烟酰胺释放试验中测量NAD糖水解酶(NADase)酶活性。通过阴离子交换色谱法纯化猪脑中的粗NADase组分。每个测定孔含有0.5μg纯化酶和浓度为18.55μM(KM的70%)的NAD。 微软稳定性。[4] 使用大鼠肝微粒体评估体外代谢稳定性。浓度为10μM的化合物在37°C下与大鼠肝微粒体(1 mg蛋白质/mL)一起孵育,并在0、5、15、30和60分钟后通过HPLC/MS进行定量。对照孵育不含微粒体。 细胞色素P450抑制试验。[4] 如前所述,使用与荧光底物孵育的重组人同工酶3A4、2D6、1A2、2C9和2C19,在384孔微孔板格式中进行细胞色素P450测定。 |
| 动物实验 |
In Vivo Pharmacokinetic Analysis. [4]
C57bl/6J mice were dosed intravenously (iv) or by oral gavage with 10 mg/kg of compound 1 (selisistat) or 35 in phosphate-buffered saline containing 4% DMSO and 10% cyclodextrin. Plasma was collected at 5, 15, 30, 60, and 90 min and 2, 4, 6, 8, and 24 h after dosing. Samples were analyzed by LCMS at Absorption Systems. Plasma samples were prepared by solid-phase extraction in a 96-well plate format. A 50-μL aliquot of plasma was combined with 300 μL of 1% phosphoric acid spiked with an internal standard (warfarin at 50 ng/mL). Plasma samples were transferred to a Waters Oasis HLB 30 mg extraction plate, washed with 5% methanol/water, and eluted with acetonitrile. The elute was evaporated to dryness under N2 at 37 °C and redissolved in 20% aqueous acetonitrile. |
| 药代性质 (ADME/PK) |
PK results [Br J Clin Pharmacol. 2015 Mar;79(3):477-91.]
Single oral doses of 5 to 600 mg selisistat were rapidly absorbed by male subjects in the fasted condition, although the rate of absorption appeared to be dose-dependent with a median tmax of selisistat increasing from 1 h post-dose at 5 mg to 4 h post-dose at 600 mg (Figure 1). Elimination of selisistat occurred in a biphasic manner, with an apparent terminal plasma half-life that appeared to increase with dose (mean values ranging from 1.6 h at 5 mg to 6.1 h at 600 mg). The AUC(0,∞) of selisistat increased in a dose proportional manner over the 5 to 300 mg dose range, with a marked increase in supra-proportionality between the 300 and 600 mg dose levels, suggesting that one or more clearance mechanisms are approaching saturation at higher doses (Figure 2A and Table 2). The fraction of unchanged drug excreted in the urine with respect to dose was low for all dose levels in male subjects, with <0.02% being eliminated up to 24 h post-dose at each dose level. Following multiple dosing, the fraction of the dose excreted in the urine remained low, but increased with time, consistent with the plasma accumulation observed. Food had a minimal effect on the single dose pharmacokinetics of selisistat in male subjects. Following a high fat breakfast, the rate of absorption was delayed, whereas the extent of absorption was largely unchanged. The multiple oral dose pharmacokinetics of selisistat showed no dose or time dependency in tmax or apparent terminal half-life. At each dose level, the morning trough selisistat plasma concentrations for individual subjects showed that steady-state was generally achieved by day 4. Consistent with the single dose finding, a supra-proportional increase in steady-state AUC(0,τ) was observed across the 100 mg once daily to 300 mg once daily range (Figure 2B), whilst the steady-state Cmax increased in a dose-proportional manner. Furthermore, the steady-state AUC(0,τ) was approximately two-fold higher for the 100 mg twice daily dose level as compared with the 100 mg once daily dose level (Table 3). In the single dose phase, between-subject variability (%CV) in terms of AUC(0,∞) and Cmax was 35–71% and 23–46%, respectively. Across all dose levels, the pooled between-subject variability for AUC(0,∞) and Cmax was 56% and 33%, respectively. In the multiple dose phase, between-subject variability (%CV) was 17–59% in males and 28–68% in females. Systemic exposure following both single and multiple dosing was higher in females than in males. AUC(0,∞), AUC(0,τ) and Cmax values were 1.1-fold, 2.2–2.3-fold and 1.7–1.9-fold higher in females than inmale subjects. There were no differences in systemic exposure or pharmacokinetic parameter estimates between Caucasian and non-Caucasians subjects. |
| 毒性/毒理 (Toxicokinetics/TK) |
Safety [Br J Clin Pharmacol. 2015 Mar;79(3):477-91.]
There were no serious adverse events reported during the study and no subjects were withdrawn due to adverse events. Single oral doses of selisistat were considered to be safe and well tolerated by healthy male subjects when administered at doses up to 600 mg, and by female subjects when administered at a dose of 300 mg selisistat (Table 5). Multiple oral doses of selisistat were also considered to be safe and well tolerated by healthy male subjects at doses up to 300 mg once daily for 7 days and by healthy female subjects when administered doses of 100 mg twice daily for 7 days. There was a low incidence of drug related adverse events in male subjects, with no increase in the number of subjects experiencing adverse events with increasing dose of selisistat. The incidence of adverse events did not exceed that observed in the placebo group. No increase in the number of adverse events reported was observed following administration of multiple doses of selisistat compared with single doses (Table 6). The majority of adverse events reported by male and female subjects were mild in severity and resolved without treatment. Only one adverse event graded as severe in intensity occurred during the study. One 18-year-old male subject experienced an episode of postural syncope 1 h and 18 min after dosing at 150 mg. This event was considered possibly related to the study drug by the investigator. Dietary state had no effect on adverse events. Following single oral doses of selisistat, the most frequent drug-related adverse event was headache, experienced by 12% of male subjects and 83% of female subjects. Following multiple oral doses of selisistat, the incidence of adverse events was low in male subjects. In female subjects, three out of six subjects reported at least one incident of gastrointestinal complaint. Overall, adverse events were more frequently reported in females than in males on drug and on placebo (Tables 5 and 6). There were no dose- or treatment-related trends in terms of clinical laboratory evaluations, including liver function tests, haematological parameters, vital signs or cardiac function. Specifically, no treatment or dose-related trends in parameters recorded on 12-lead safety ECGs were noted and there were no clinically relevant findings in the ECG morphology at any dose level of selisistat. There were no subjects with a QTc interval >480 ms or an increase from baseline >60 ms as assessed from the 12-lead safety ECGs. There were no clinically significant findings in physical examinations, postural control or neurological examinations and no changes in sway platform performance. Concentration−effect modelling of ECG parameters [Br J Clin Pharmacol. 2015 Mar;79(3):477-91.] The variability of the QTc data measured as the standard deviation of the between-subject ΔQTcF was low, 5.3 ms and 6.8 ms in the single ascending dose (SAD) and multiple ascending dose (MAD) parts, respectively 15. The change from baseline QTcF across dose groups in part 1, in which the highest plasma concentrations were achieved, is shown in Table 7. The pattern across time points and dose groups did not suggest a dose-dependent effect of selisistat on the QTc interval. No significant concentration-dependent effect on ΔΔQTcF was seen after single doses from 5 mg to 600 mg of selisistat within the observed plasma concentration range. A linear model with an intercept provided an acceptable fit of the data and the estimated population intercept and slope were 0.9 ms (90% CI −0.2, 2.0) and −0.00026 ms per ng ml−1 (90% CI −0.00063, 0.00010), respectively (Figure 3A). The analysis of data from the MAD part provided similar results with an intercept of 2.8 ms (90% CI −0.16, 5.71) and an estimated slope of −0.00011 ms per ng ml−1 (90% CI −0.00087, 0.00066; Figure 3B). The ΔΔQTcF effect at the observed geometric Cmax of 26.6 μm after a single dose of 600 mg using this model can be predicted to −0.9 ms (90% CI −3.3, 1.4). A ΔΔQTcF effect of approximately 2.8 ms (90% CI −0.1, 5.6) can be predicted for the observed Cmax level of 22.5 μm after 7 days of dosing of 300 mg once daily. For plasma concentrations exceeding the mean Cmax level, e.g. 30 μm, a QTcF effect of 3.7 ms (90% CI −0.1, 7.5) can be predicted using the same model. The upper bound of the 90% CI of the projected ΔΔQTcF effect was below 10 ms for all plasma concentrations observed in both the SAD and the MAD part of the study (Figure 3A, B). |
| 参考文献 |
|
| 其他信息 |
(S)-selisistat is a 6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide that has S configuration It is the active enantiomer. It has a role as a Sir1 inhibitor. It is an enantiomer of a (R)-selisistat.
Human SIRT1 is an enzyme that deacetylates the p53 tumor suppressor protein and has been suggested to modulate p53-dependent functions including DNA damage-induced cell death. In this report, we used EX-527, a novel, potent, and specific small-molecule inhibitor of SIRT1 catalytic activity to examine the role of SIRT1 in p53 acetylation and cell survival after DNA damage. Treatment with EX-527 dramatically increased acetylation at lysine 382 of p53 after different types of DNA damage in primary human mammary epithelial cells and several cell lines. Significantly, inhibition of SIRT1 catalytic activity by EX-527 had no effect on cell growth, viability, or p53-controlled gene expression in cells treated with etoposide. Acetyl-p53 was also increased by the histone deacetylase (HDAC) class I/II inhibitor trichostatin A (TSA). EX-527 and TSA acted synergistically to increase acetyl-p53 levels, confirming that p53 acetylation is regulated by both SIRT1 and HDACs. While TSA alone reduced cell survival after DNA damage, the combination of EX-527 and TSA had no further effect on cell viability and growth. These results show that, although SIRT1 deacetylates p53, this does not play a role in cell survival following DNA damage in certain cell lines and primary human mammary epithelial cells. [1] Sepsis is defined as a systemic inflammatory response syndrome that disorders the functions of host immune system, including the imbalance between pro- and anti-inflammatory responses mediated by immune macrophages. Sepsis could also induce acute hyperglycemia. Studies have shown that the silent mating type information regulation 2 homolog 1 (SIRT1), an NAD+-dependent deacetylase, mediates NF-κb deacetylation and inhibits its function. Therefore, SIRT1 is likely to play an important role in high glucose-mediated inflammatory signalings. Here we demonstrate that high glucose significantly downregulates both the mRNA and protein levels of SIRT1 and upregulates the mRNA level and the release of two pro-inflammatory cytokines, IL-1β and TNF-α, in RAW264.7 macrophages. Interestingly, the reduced level of SIRT1 by high glucose is remarkably upregulated by SIRT1 activator SRT1720, while the level and the release of IL-1β and TNF-α significantly decrease with the use of SRT1720. However, when the function of SIRT1 is inhibited by EX527 or its expression is suppressed by RNAi, the upregulated level and release of IL-1β and TNF-α by high glucose are further increased. Taken together, these findings collectively suggest that SIRT1 is an important regulator in many high glucose-related inflammatory diseases such as sepsis. [2] Objective: Obesity, a sirtuin-1 (SIRT-1) -deficient state, increases morbidity and resource utilization in critically ill patients. SIRT-1 deficiency increases microvascular inflammation and mortality in early sepsis. The objective of the study was to study the effect of resveratrol (RSV), a SIRT-1 activator, on microvascular inflammation in obese septic mice. Methods: ob/ob and C57Bl/6 (WT) mice were pretreated with RSV versus dimethyl sulfoxide (DMSO) (vehicle) prior to cecal ligation and puncture (sepsis). We studied (1) leukocyte/platelet adhesion, (2) E-selectin, ICAM-1, and SIRT-1 expression in small intestine, and (3) 7-day survival. A group of RSV-treated mice received SIRT-1 inhibitor (EX-527) with sepsis induction, and leukocyte/platelet adhesion and E-selectin/ICAM-1 expression were studied. We treated endothelial (HUVEC) cells with RSV to study E-selectin/ICAM-1 and p65-acetylation (AC-p65) in response to lipopolysaccharide (LPS). Results: RSV treatment decreased leukocyte/platelet adhesion and E-selectin/ICAM-1 expression with increased SIRT-1 expression in septic ob/ob and WT mice, decreased E-selectin/ICAM-1 expression via increased SIRT-1 expression, and decreased AC-p65 expression in HUVEC. EX-527 abolished RSV-induced attenuation of microvascular inflammation in ob/ob septic mice. Finally, ob/ob mice in the sepsis+RSV group had significantly increased 7-day survival versus the sepsis+vehicle group. Conclusions: RSV increases SIRT-1 expression in ob/ob septic mice to reduce microvascular inflammation and improves survival. [3] High-throughput screening against the human sirtuin SIRT1 led to the discovery of a series of indoles as potent inhibitors that are selective for SIRT1 over other deacetylases and NAD-processing enzymes. The most potent compounds described herein inhibit SIRT1 with IC50 values of 60-100 nM, representing a 500-fold improvement over previously reported SIRT inhibitors. Preparation of enantiomerically pure indole derivatives allowed for their characterization in vitro and in vivo. Kinetic analyses suggest that these inhibitors bind after the release of nicotinamide from the enzyme and prevent the release of deacetylated peptide and O-acetyl-ADP-ribose, the products of enzyme-catalyzed deacetylation. These SIRT1 inhibitors are low molecular weight, cell-permeable, orally bioavailable, and metabolically stable. These compounds provide chemical tools to study the biology of SIRT1 and to explore therapeutic uses for SIRT1 inhibitors. [4] |
| 分子式 |
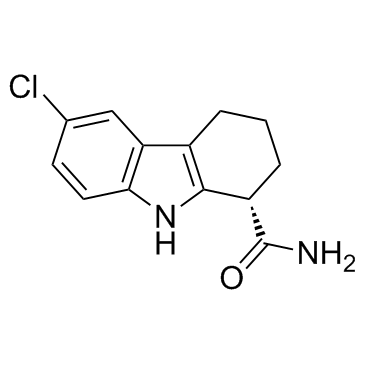
C13H13CLN2O
|
|---|---|
| 分子量 |
248.71
|
| 精确质量 |
248.071
|
| 元素分析 |
C, 62.78; H, 5.27; Cl, 14.25; N, 11.26; O, 6.43
|
| CAS号 |
848193-68-0
|
| 相关CAS号 |
Selisistat;49843-98-3;(R)-Selisistat;848193-69-1
|
| PubChem CID |
707029
|
| 外观&性状 |
Off-white to light yellow solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
531.7±38.0 °C at 760 mmHg
|
| 闪点 |
275.4±26.8 °C
|
| 蒸汽压 |
0.0±1.4 mmHg at 25°C
|
| 折射率 |
1.688
|
| LogP |
2.22
|
| tPSA |
59.87
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
17
|
| 分子复杂度/Complexity |
323
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C1C[C@@H](C2=C(C1)C3=C(N2)C=CC(=C3)Cl)C(=O)N
|
| InChi Key |
FUZYTVDVLBBXDL-VIFPVBQESA-N
|
| InChi Code |
InChI=1S/C13H13ClN2O/c14-7-4-5-11-10(6-7)8-2-1-3-9(13(15)17)12(8)16-11/h4-6,9,16H,1-3H2,(H2,15,17)/t9-/m0/s1
|
| 化学名 |
(1S)-6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide
|
| 别名 |
EX 527(S); 848193-68-0; (S)-selisistat; (1S)-6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide; (S)-6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide; Selisistat, (S)-; Selisistat S-enantiomer; MUD9R3TJV3; EX-527(S);(S)-Selisistat EX-527(S)
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 100 mg/mL (~402.07 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (10.05 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (10.05 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (10.05 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.0207 mL | 20.1037 mL | 40.2075 mL | |
| 5 mM | 0.8041 mL | 4.0207 mL | 8.0415 mL | |
| 10 mM | 0.4021 mL | 2.0104 mL | 4.0207 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04184323 | Withdrawn | Drug: EX-527 (Selisistat) Drug: Placebo |
Endometriosis Uterine Diseases |
Wake Forest University Health Sciences | January 2022 | Phase 2 |