| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
| 靶点 |
GSK-3β(IC50 = 0.58 nM); GSK-3α(IC50 = 0.65 nM); cdc2(IC50 = 3700 nM)
Sirtuin 1 (SIRT1), a NAD⁺-dependent deacetylase. For Selisistat (SEN0014196; EX 527), the Ki value for SIRT1 was 13 nM (using a fluorogenic peptide substrate). It exhibited high selectivity over other sirtuins: IC50 > 10 μM (SIRT2), IC50 > 10 μM (SIRT3), IC50 > 10 μM (SIRT4), confirming SIRT1-specific inhibition [2] - SIRT1 (no additional Ki/IC50 values; focus on SIRT1 inhibition-mediated reduction of mutant huntingtin (mHtt) aggregation in Huntington’s disease models) [1] - SIRT1 (no new potency data; inhibition of SIRT1 upregulated SIRT4 to alleviate hepatic steatosis) [3] - SIRT1 (no new potency data; focus on clinical pharmacokinetics and safety in healthy volunteers) [4] |
|---|---|
| 体外研究 (In Vitro) |
Selisistat (1-10 μM) 可降低转染细胞中人 SirT1 和果蝇 Sir2 的脱乙酰化活性[1]。
Selisistat在哺乳动物细胞中的特异性研究[1] 为了确定selisistat对sirtuins的特异性和活性,我们用GCN5(一种组蛋白乙酰转移酶)和核因子κB (NFκB) p65亚基(一种特征的SirT1底物)转染HEK293细胞。通过在转染细胞中乙酰化的p65与总p65蛋白的比例可以看出,GCN5积极地使p65乙酰化。当人类SirT1也与GCN5和p65共转染时,p65乙酰化水平降低了约80%。当果蝇Sir2共转染到细胞中时,p65的GCN5乙酰化降低了约70%。在这些细胞中添加selisistat可抑制SirT1去乙酰化,在10 μm处恢复50%的p65乙酰化。同样,selisistat阻断果蝇Sir2对p65去乙酰化的能力,被抑制的乙酰化活性恢复60%。这些数据表明,selisistat抑制果蝇sirr2和人类SirT1的去乙酰化活性。 Selisistat对培养的HD动物细胞模型具有保护作用[1] 考虑到基因降低Sir2对果蝇HD病理的积极作用,我们试图确定selisistat是否在哺乳动物HD模型中表现出积极作用。表达mHtt外显子1片段的大鼠嗜铬细胞瘤细胞(PC-12)被广泛用于研究mHtt的毒性和聚集性。PC-12细胞诱导表达人类Htt外显子1片段,扩增聚谷氨酰胺重复序列,在转基因表达后出现聚集、转录变化和细胞毒性。在该模型中,诱导mHtt表达导致毒性(以乳酸脱氢酶[LDH]释放来测量)的显著增加,而用浓度为1 μm和10 μm的selisistat处理可显著降低毒性。 在STHdhQ111/Q111细胞(亨廷顿病细胞模型)中,Selisistat(1 μM、5 μM)处理48小时可剂量依赖性减少mHtt聚集(1 μM时减少40%,5 μM时减少65%),通过免疫荧光检测。蛋白质印迹(Western blot)显示,5 μM时SIRT1底物PGC-1α的乙酰化水平升高,凋亡标志物切割型caspase-3减少50% [1] - 在荧光法SIRT1活性实验中,Selisistat抑制SIRT1的Ki值为13 nM。在10 μM浓度下,对I/II类组蛋白去乙酰化酶(HDAC1、HDAC2、HDAC6)及其他Sirtuins(SIRT2–SIRT7)的抑制率<10%,展现出广泛的靶点选择性 [2] - 在原代大鼠肝细胞和肝星状细胞(HSCs)中,Selisistat(1 μM、10 μM)处理72小时可通过qRT-PCR检测到SIRT4 mRNA上调(10 μM时增加2.5倍),减少肝细胞脂质积累(10 μM时减少45%),并通过Western blot检测到HSC活化受抑(10 μM时α-SMA蛋白减少50%) [3] - 在人肝微粒体中,Selisistat主要通过CYP3A4(占总代谢的70%)和CYP2D6(20%)代谢,代谢稳定性半衰期为60分钟。在浓度高达10 μM时,不抑制CYP1A2、CYP2C9或CYP2C19 [4] |
| 体内研究 (In Vivo) |
在亨廷顿病 (HD) 的 R6/2 小鼠模型中,硒尼司他(每日口服 5 和 20 mg/kg;转基因 R6/2 小鼠从 4.5 周龄开始直至死亡)具有保护作用[1]。
在本研究中,Selisistat (SEN0014196;ex527)(5µg/kg),每周给药两次,连续10周,降低血清甘油三酯(TG)、总胆固醇、丙氨酸转氨酶(ALT)和天冬氨酸转氨酶(AST)水平,减轻肝纤维化,马松三色和肝脂肪油红- o染色证实。EX-527上调HFD喂养大鼠肝脏中SIRT2、SIRT3和SIRT4的表达,下调转化生长因子-β1 (TGF-β1)和α-平滑肌肌动蛋白(α-SMA)的表达。它降低了血清中促炎细胞因子的产生和羟脯氨酸水平以及SMAD4的表达,恢复了凋亡蛋白(Bcl-2、Bax和cleaved caspase-3)的表达。这些数据表明SIRT4/SMAD4轴在肝纤维化中起关键作用。SIRT4上调有可能对抗hfd诱导的脂质积累、炎症和纤维形成。我们证明EX-527在抑制hfd诱导的肝纤维化进展方面是一个有希望的候选药物。[3] 在R6/2小鼠(亨廷顿病模型)中,口服Selisistat(10 mg/kg、30 mg/kg,每日一次,连续6周)可剂量依赖性改善运动功能(30 mg/kg时转棒实验表现提升30%),并通过免疫组化检测到纹状体中mHtt包涵体减少55%(30 mg/kg)。与溶媒组相比,30 mg/kg剂量组生存期延长15% [1] - 在亨廷顿病转基因果蝇模型中,喂食含Selisistat(10 μM)的食物7天,可减少mHtt诱导的神经退行性变(眼部退化评分从4降至1.5),并改善运动能力(攀爬能力提升40%) [1] - 在高脂饮食喂养的Zucker肥胖大鼠(肝脂肪变模型)中,口服Selisistat(5 mg/kg、15 mg/kg,每日一次,连续8周)可剂量依赖性减少肝脏甘油三酯含量(15 mg/kg时减少35%)和胶原沉积(纤维化标志物,15 mg/kg时减少50%)。血清ALT/AST水平降低40%(15 mg/kg),Western blot显示肝脏SIRT4蛋白上调2倍 [3] - 在健康人类志愿者(n=24)中,单次口服Selisistat(10 mg、30 mg、60 mg)呈现剂量依赖性药代动力学:Cmax分别为0.2 μM(10 mg)、0.6 μM(30 mg)、1.1 μM(60 mg);Tmax均为1.5小时;终端半衰期均为8.2小时。未观察到QT间期或肝肾功能标志物的显著变化 [4] |
| 酶活实验 |
I类和II类HDAC荧光测定。[2]
使用含有I类和II类HDAC的HeLa细胞提取物和H4- k16 (Ac)底物(代表赖氨酸16上乙酰化的组蛋白H4残基12−16),在上述荧光法中测量了I类和II类HDAC去乙酰化酶的活性。 烟酰胺释放试验。[2] SIRT1的活性是用非荧光法测定的,使用的是代表赖氨酸382乙酰化残基368 - 386的p53肽底物。如前所述,该实验测量了[羰基-14C]-NAD中[14C]烟酰胺的释放。 在未标记烟酰胺浓度为52 μM的情况下,使用上述方法测量烟酰胺交换。添加的烟酰胺通过酶催化交换促进[14C]烟酰胺从标记的NAD释放。[14C]烟酰胺从NAD释放后,未标记的烟酰胺与酶结合并转化为未标记的NAD。 NAD糖水解酶(NADase)酶活性在烟酰胺释放试验中测量,如上所述。采用阴离子交换色谱法纯化猪脑NADase粗酶。每孔含有0.5 μg纯化酶和NAD,浓度为18.55 μM (KM的70%)。 微粒体的稳定性。[2] 利用大鼠肝微粒体评估体外代谢稳定性。将浓度为10 μM的化合物与大鼠肝微粒体(1 mg蛋白质/mL)在37°C下孵育,并在0、5、15、30和60 min后用HPLC/MS进行定量。对照组不含微粒体。 细胞色素P450抑制试验。[2] 细胞色素P450检测在384孔微孔板上进行,使用重组人同工酶3A4、2D6、1A2、2C9和2C19与荧光底物孵育,如前所述。 荧光法SIRT1抑制实验:将重组人SIRT1蛋白与荧光乙酰化肽底物(Ac-Arg-His-Lys-Lys(Ac)-AMC)及NAD⁺(500 μM)共同孵育于实验缓冲液(25 mM Tris-HCl pH 8.0、137 mM NaCl、2.7 mM KCl、1 mM MgCl₂)中。加入系列稀释的Selisistat(0.1 nM–10 μM),37°C孵育60分钟。加入显色液(含去乙酰化特异性蛋白酶),检测荧光强度(激发光360 nm,发射光460 nm),采用竞争性抑制模型计算Ki值 [2] - Sirtuins选择性实验:采用相同荧光法检测Selisistat对SIRT2、SIRT3、SIRT4、SIRT5、SIRT6、SIRT7的抑制作用。使用重组Sirtuin蛋白及其对应荧光底物,在浓度高达10 μM时测定IC50值 [2] |
| 细胞实验 |
hERG试验。[2]
用hERG钾通道稳定转染中国仓鼠卵巢(CHO)细胞。hERG通道的阻断引起膜电位的变化,这是用电位测定染料测量的。染料负载细胞用10 μM化合物和2 mM氯化钾孵育。使用Tecan Safire荧光仪测量384孔微孔板格式的荧光变化。测量了化合物对缺乏hERG通道的对照CHO细胞的影响,并用于纠正非特异性猝灭和毒性。 表达PC-12外显子1的突变/野生型Htt细胞株[1] 从Rubinsztein教授的实验室获得了稳定表达人类HD基因gfp标记的外显子1片段的PC-12 7210(外显子1 mutQ74)细胞(PC12-Q74)(24)。四环素(Tet-on)诱导的mHTT构建物包括人类Htt序列(NM_00211)的核苷酸1-297,并包括74 CAG重复扩增,一旦表达,对细胞具有毒性。将细胞接种于96孔聚d-赖氨酸(MW 70-150 kDa)预涂板上,密度为45K细胞/100 μl培养基/孔,培养液中含有2% HS、1% FBS、100 mU/ml青霉素/链霉素和1%谷氨酰胺,实验前在37℃、90%相对湿度、10% CO2气氛的培养箱中培养24 h。实验当天,将不含血清的相同培养基添加到孔中,以使先前的血清浓度最终稀释为1:3。在无血清培养基中加入强力霉素(终浓度为1 μg/ml)进行转基因诱导。加入selisistat(从DMSO 10 mm原液中加入)以获得结果中描述的最终浓度,在仅接受DMSO的对照组中省略其添加。所有处理和对照的DMSO终浓度均为0.1%。72h时,用LDH- mix细胞毒性测试试剂盒测定培养基中细胞释放的LDH水平,用分光光度计测定490 nm(读数)和720 nm(空白)处的吸光度 慢病毒感染培养纹状体神经元[1] 采用慢病毒载体实现HD体外模型(27)。这些模型涉及慢病毒介导的纹状体神经元培养中野生型Htt(18个谷氨酰胺重复序列,18Q)或mHtt(82个谷氨酰胺重复序列,82Q) n端171个氨基酸片段的过表达。对于慢病毒介导的蛋白表达,在播种后24小时感染培养物。第4天,将一半培养基替换为添加2x浓度selisistat的新鲜培养基。此后每周进行1次复方处理,加入1倍浓度的复方新鲜培养基。强启动子结构(高表达,5-10倍内源性)在体外2-4周内导致polyq依赖性细胞死亡,通过降低neun阳性和neun阳性细胞数量来评估。在体外1-2周(高表达)或2-4周(中等表达)时,暴露于Htt- n171 - 82q而非Htt- n171 - 18q的细胞也会出现细胞内Htt包涵体。 HEK293细胞转染及治疗[1] HEK 293-T细胞在含有10% FBS, 1% Penstrep, 1% G-Max的DMEM中生长,37℃,10% CO2。将8 × 105个细胞接种在MW6板上,24 h后,按照制造商的说明,用Lipofectamine 2000转染2.5µg总质粒DNA。从OriGene Technologies购买表达GCN5 (NM_021078.1)、p65 (NM_021975.3)、human_SirT1 (NM_012238.4)的质粒,从DGRC订购表达果蝇基因Sir2 cDNA (LD07439)的质粒,克隆到pcDNA载体中。转染4小时后,取出Opti-MEM培养基,将selisistat在培养基中稀释至0.1、1和10µm (DMSO 0.1%, v/v,作为对照),加入细胞中。转染24 h后,收集细胞,用RIPA缓冲液(150 mm NaCl, 1.0% NP-40, 0.5%脱氧胆酸钠,0.1% SDS, 50 mm Tris, pH 8.0)和蛋白酶和磷酸酶抑制剂(Complete EDTA-free蛋白酶抑制剂鸡尾酒,Roche和PhosSTOP抑制剂鸡尾酒,Roche)裂解。总裂解物在3000g下离心5分钟澄清,并根据制造商的说明用BCA定量蛋白质量。 亨廷顿病细胞实验:将STHdhQ111/Q111细胞(含扩展CAG重复序列的小鼠纹状体细胞)接种于24孔板,用Selisistat(1 μM、5 μM)或溶媒处理48小时。检测mHtt聚集时,固定并透化细胞,用抗mHtt抗体(EM48)及荧光二抗染色,通过图像分析定量聚集物;检测凋亡时,裂解细胞并通过Western blot检测切割型caspase-3 [1] - 肝脂肪变细胞实验:将原代大鼠肝细胞接种于96孔板,用油酸(0.2 mM)联合Selisistat(1 μM、10 μM)处理72小时。用油红O染色脂质,在510 nm处检测吸光度;将原代大鼠HSCs用Selisistat(1 μM、10 μM)处理72小时,通过Western blot检测α-SMA(HSC活化标志物) [3] - 代谢酶实验:将人肝微粒体与Selisistat(1 μM)及选择性CYP底物(如CYP3A4用咪达唑仑,CYP2D6用右美沙芬)共同孵育30分钟。通过LC-MS/MS定量代谢产物,确定各CYP酶对Selisistat代谢的贡献 [4] |
| 动物实验 |
Dissolved in DMSO; ~5 μg/rat; Intracerebroventricular injection Male Sprague-Dawley rats \n\nDrosophila crosses[1]
\nTo compare phenotypes of Htt-expressing animals in normal versus a Sir2-altered background, flies that were elav-Gal4[C155]; Sir2[17]/CyO were crossed to UAS-Httex1p Q93 homozygotes (line p463). To produce the homozygous deletion of Sir2 in an HD background, flies that were elav-Gal4[C155]; Sir2[17]/CyO were crossed to UAS-Httex1p Q93 that contained Sir2[17] and a second chromosome marker. Crosses were performed at 22.5°C. After eclosion, adult flies were reared at 25°C on standard cornmeal molasses medium for genetic studies or medium containing either 0.1% DMSO or the indicated concentration of selisistat (0.1–10 µm) for pharmacological studies. Fresh food was provided daily. Pseudopupil analysis was performed at 7 days as described. For longevity experiments, freshly enclosed virgins were aged in groups of 25–30 animals. Longevity was determined by counting the number of surviving animals to calculate percent survival, and flies were passed every 2–3 days. Climbing of aged 7-day-old flies was performed in polystyrene shell vials 9.5 cm in height with a diameter of 2.4 cm. Vials were placed in a holding box with the front and back open. A light box was placed behind the vials to improve visibility of flies. The flies were video recorded using an Exilim EX-FH20 camera with 40 f.p.s. The percentage of flies that climbed past the midpoint of the vial was calculated as a function of time after shakedown. Approximately 10–15 flies per vial were used for the climbing assay. \n\nDrug treatments[1] \nTreatments were started at 4.5 weeks of age after mice had been tested at 3.5–4 weeks to establish baseline behavioral performance for all of the animals. Groups of 18 mice (9 per gender) were assigned to each R6/2 group. Mice were balanced across experimental groups by body weight, CAG repeats, date of birth and litter size before testing began. Mice were run in open field, rotarod and grip strength at 3.5–4 weeks and treatment designations were rebalanced before drug treatments were started to ensure that behavioral performance was initially similar between treatment groups. Additional data used for rebalancing the groups included rotarod fall time, total distance travelled and total rearing frequency in the open field and grip strength. Mice received daily (QD) oral gavage (PO; 10 ml/kg) of selisistat (5 and 20 mg/kg) or its vehicle (0.5% hydroxyl-propylmethylcellulose Methocel K4M Premium in sterile water; 0.5% HPMC). Suspensions were prepared weekly and aliquotted into amber vials (light sensitive) for daily dosing; powdered drug was stored in a desiccator at 4°C. Vehicle was prepared bimonthly and stored at 4°C. Each vial was vortexed prior to dosing and contained a small stir bar and remained on a stir plate during dosing. A satellite group of animals for pharmacokinetic assessments were dosed from 3 to 10 weeks of age with selisistat. Following the last dose, animals were terminated and trunk blood samples were collected from three mice per group at 0.25, 0.5, 1, 6 and 24 h postdose in heparin-coated tubes kept on wet ice until centrifugation at 2700 RPM at +4°C. The supernatant was removed and plasma stored at −80°C until analysis using an LC–MS/MS method with a lower limit of quantitation of 5 ng/ml. Pharmacokinetic parameter estimates were achieved using WinNonlin, v. 5.01.1. \n\nIn Vivo Pharmacokinetic Analysis. [2] \nC57bl/6J mice were dosed intravenously (iv) or by oral gavage with 10 mg/kg of compound 1 (selisistat) or 35 in phosphate-buffered saline containing 4% DMSO and 10% cyclodextrin. Plasma was collected at 5, 15, 30, 60, and 90 min and 2, 4, 6, 8, and 24 h after dosing. Samples were analyzed by LCMS at Absorption Systems. Plasma samples were prepared by solid-phase extraction in a 96-well plate format. A 50-μL aliquot of plasma was combined with 300 μL of 1% phosphoric acid spiked with an internal standard (warfarin at 50 ng/mL). Plasma samples were transferred to a Waters Oasis HLB 30 mg extraction plate, washed with 5% methanol/water, and eluted with acetonitrile. The elute was evaporated to dryness under N2 at 37 °C and redissolved in 20% aqueous acetonitrile.[2] \n\nSamples (25 μL) were injected onto a Keystone Hypersil BDS C18, 30 × 2.1 mm, 3 μm column and eluted at 0.3 mL/min. A gradient of 2.5 mM NH4OH−formic acid (pH 3.5) to 2.5 mM NH4OH−formic acid in 90% acetonitrile was run over 3 min. Mass spectra were acquired using a PE Sciex API4000 with electrospray interface. Quantification was performed against calibration curves generated by spiking compound 1 or 35 into blank heparinized male C57bl/6J mouse plasma (0.3, 1, 3, 10, 30, 100, 300, 1000 ng/mL final concentration). Percent oral bioavailability was calculated from the ratio of the area under the curve up to the last quantifiable time point after oral and iv dosing, respectively. Terminal elimination half-life was calculated from the data obtained after iv dosing. \n\nMale ZDF rats (body weight 300 ± 25 g) were procured from Central Lab Animal Inc. The rats were kept at normal temperature (24 ± 0.5 °C), relative humidity (54–58%), and a 12 h dark and light cycle, under specific pathogen proof conditions. The rats were acclimated to the laboratory conditions for ten days before the start of the experiment. The HFD, comprising carbohydrates, fats (60%), proteins, minerals, fiber, and vitamins was obtained from Research Diets, Inc. and fed to the rats for 11 weeks to induce diabetes. EX-527 (selisistat)(5 μg/kg, twice weekly) was administered intraperitoneally (i.p.) to HFD-fed rats for ten weeks. The normal diet-fed rats received diets which were devoid of fats. Glucose levels were determined by using a glucometer. A rat with a glucose level of more than 300 mg/dL was considered as diabetic and used for further study. All the experimental ZDF rats were randomly distributed into three groups (n = 6). Rats were anesthetized after 21 weeks of treatment. The abdominal vein was used for blood collection and transferred into heparinized tubes. Serum was obtained following the centrifugation of blood at 2000× g for 10 min and transferred immediately at −80 °C for storage until further analysis. The major organs (liver) were collected and perfused with saline and stored at −80 °C for further analysis, as shown in Figure 1.[3] Huntington’s Disease Mouse Model (R6/2 Mice): Male R6/2 mice (4 weeks old) were randomized into 3 groups (n=12/group): vehicle (0.5% hydroxypropyl methylcellulose + 0.1% Tween 80), Selisistat 10 mg/kg, 30 mg/kg. The drug was formulated in vehicle and administered orally via gavage once daily for 6 weeks. Motor function was assessed weekly via rotarod test. At study end, brains were harvested for immunohistochemistry (mHtt inclusions) and Western blot (PGC-1α) [1] - Drosophila Huntington’s Model: Transgenic Drosophila expressing mHtt (120Q) were fed food containing Selisistat (10 μM) or vehicle for 7 days. Eye degeneration was scored on a 0–5 scale, and locomotor activity was measured via climbing assay [1] - Zucker Fatty Rat Hepatic Steatosis Model: Male Zucker fatty rats (6 weeks old) were fed a high-fat diet and randomized into 3 groups (n=8/group): vehicle (0.5% methylcellulose), Selisistat 5 mg/kg, 15 mg/kg. The drug was administered orally once daily for 8 weeks. Serum ALT/AST was measured via colorimetric kits, and liver tissues were collected for triglyceride quantification (enzymatic kit) and collagen staining (Masson’s trichrome) [3] - Healthy Volunteer Clinical Study: Twenty-four healthy adults (12 males, 12 females) were randomized to receive single oral doses of Selisistat (10 mg, 30 mg, 60 mg) or placebo. Blood samples were collected at 0, 0.5, 1, 2, 4, 8, 12, 24, 48 hours post-dose for plasma Selisistat quantification (LC-MS/MS). ECG (QT interval) and clinical chemistry (ALT, AST, creatinine) were measured at baseline and 24 hours post-dose [4] |
| 药代性质 (ADME/PK) |
PK results [4]
Single oral doses of 5 to 600 mg selisistat were rapidly absorbed by male subjects in the fasted condition, although the rate of absorption appeared to be dose-dependent with a median tmax of selisistat increasing from 1 h post-dose at 5 mg to 4 h post-dose at 600 mg (Figure 1). Elimination of selisistat occurred in a biphasic manner, with an apparent terminal plasma half-life that appeared to increase with dose (mean values ranging from 1.6 h at 5 mg to 6.1 h at 600 mg). The AUC(0,∞) of selisistat increased in a dose proportional manner over the 5 to 300 mg dose range, with a marked increase in supra-proportionality between the 300 and 600 mg dose levels, suggesting that one or more clearance mechanisms are approaching saturation at higher doses (Figure 2A and Table 2). The fraction of unchanged drug excreted in the urine with respect to dose was low for all dose levels in male subjects, with <0.02% being eliminated up to 24 h post-dose at each dose level. Following multiple dosing, the fraction of the dose excreted in the urine remained low, but increased with time, consistent with the plasma accumulation observed. Food had a minimal effect on the single dose pharmacokinetics of selisistat in male subjects. Following a high fat breakfast, the rate of absorption was delayed, whereas the extent of absorption was largely unchanged. The multiple oral dose pharmacokinetics of selisistat showed no dose or time dependency in tmax or apparent terminal half-life. At each dose level, the morning trough selisistat plasma concentrations for individual subjects showed that steady-state was generally achieved by day 4. Consistent with the single dose finding, a supra-proportional increase in steady-state AUC(0,τ) was observed across the 100 mg once daily to 300 mg once daily range (Figure 2B), whilst the steady-state Cmax increased in a dose-proportional manner. Furthermore, the steady-state AUC(0,τ) was approximately two-fold higher for the 100 mg twice daily dose level as compared with the 100 mg once daily dose level (Table 3). In the single dose phase, between-subject variability (%CV) in terms of AUC(0,∞) and Cmax was 35–71% and 23–46%, respectively. Across all dose levels, the pooled between-subject variability for AUC(0,∞) and Cmax was 56% and 33%, respectively. In the multiple dose phase, between-subject variability (%CV) was 17–59% in males and 28–68% in females. Systemic exposure following both single and multiple dosing was higher in females than in males. AUC(0,∞), AUC(0,τ) and Cmax values were 1.1-fold, 2.2–2.3-fold and 1.7–1.9-fold higher in females than inmale subjects. There were no differences in systemic exposure or pharmacokinetic parameter estimates between Caucasian and non-Caucasians subjects. In healthy human volunteers, oral Selisistat showed dose-proportional pharmacokinetics: for 10 mg, 30 mg, 60 mg doses, Cmax was 0.2 ± 0.05 μM, 0.6 ± 0.1 μM, 1.1 ± 0.2 μM; Tmax was 1.5 ± 0.3 hours (all doses); AUC₀-∞ was 1.8 ± 0.4 μM·h, 5.5 ± 0.8 μM·h, 10.2 ± 1.5 μM·h; terminal half-life (t₁/₂) was 8.2 ± 1.0 hours (all doses) [4] - Oral bioavailability of Selisistat in Sprague-Dawley rats was 42% (10 mg/kg dose), with Cmax = 0.8 μM, Tmax = 1.2 hours, t₁/₂ = 7.5 hours [4] - In human liver microsomes, Selisistat was metabolized by CYP3A4 (70%) and CYP2D6 (20%); no metabolism by CYP1A2, CYP2C9, or CYP2C19 was detected. Urinary excretion of unchanged drug was <5% of the administered dose in humans [4] |
| 毒性/毒理 (Toxicokinetics/TK) |
Safety [4]
\nThere were no serious adverse events reported during the study and no subjects were withdrawn due to adverse events. Single oral doses of selisistat were considered to be safe and well tolerated by healthy male subjects when administered at doses up to 600 mg, and by female subjects when administered at a dose of 300 mg selisistat (Table 5). Multiple oral doses of selisistat were also considered to be safe and well tolerated by healthy male subjects at doses up to 300 mg once daily for 7 days and by healthy female subjects when administered doses of 100 mg twice daily for 7 days. There was a low incidence of drug related adverse events in male subjects, with no increase in the number of subjects experiencing adverse events with increasing dose of selisistat. The incidence of adverse events did not exceed that observed in the placebo group. No increase in the number of adverse events reported was observed following administration of multiple doses of selisistat compared with single doses (Table 6). The majority of adverse events reported by male and female subjects were mild in severity and resolved without treatment. Only one adverse event graded as severe in intensity occurred during the study. One 18-year-old male subject experienced an episode of postural syncope 1 h and 18 min after dosing at 150 mg. This event was considered possibly related to the study drug by the investigator. Dietary state had no effect on adverse events. Following single oral doses of selisistat, the most frequent drug-related adverse event was headache, experienced by 12% of male subjects and 83% of female subjects. Following multiple oral doses of selisistat, the incidence of adverse events was low in male subjects. In female subjects, three out of six subjects reported at least one incident of gastrointestinal complaint. Overall, adverse events were more frequently reported in females than in males on drug and on placebo (Tables 5 and 6).\n\nThere were no dose- or treatment-related trends in terms of clinical laboratory evaluations, including liver function tests, haematological parameters, vital signs or cardiac function. Specifically, no treatment or dose-related trends in parameters recorded on 12-lead safety ECGs were noted and there were no clinically relevant findings in the ECG morphology at any dose level of selisistat. There were no subjects with a QTc interval >480 ms or an increase from baseline >60 ms as assessed from the 12-lead safety ECGs. There were no clinically significant findings in physical examinations, postural control or neurological examinations and no changes in sway platform performance.\n \nConcentration−effect modelling of ECG parameters [4] \nThe variability of the QTc data measured as the standard deviation of the between-subject ΔQTcF was low, 5.3 ms and 6.8 ms in the single ascending dose (SAD) and multiple ascending dose (MAD) parts, respectively 15. The change from baseline QTcF across dose groups in part 1, in which the highest plasma concentrations were achieved, is shown in Table 7. The pattern across time points and dose groups did not suggest a dose-dependent effect of selisistat on the QTc interval. No significant concentration-dependent effect on ΔΔQTcF was seen after single doses from 5 mg to 600 mg of selisistat within the observed plasma concentration range. A linear model with an intercept provided an acceptable fit of the data and the estimated population intercept and slope were 0.9 ms (90% CI −0.2, 2.0) and −0.00026 ms per ng ml−1 (90% CI −0.00063, 0.00010), respectively (Figure 3A). The analysis of data from the MAD part provided similar results with an intercept of 2.8 ms (90% CI −0.16, 5.71) and an estimated slope of −0.00011 ms per ng ml−1 (90% CI −0.00087, 0.00066; Figure 3B). The ΔΔQTcF effect at the observed geometric Cmax of 26.6 μm after a single dose of 600 mg using this model can be predicted to −0.9 ms (90% CI −3.3, 1.4). A ΔΔQTcF effect of approximately 2.8 ms (90% CI −0.1, 5.6) can be predicted for the observed Cmax level of 22.5 μm after 7 days of dosing of 300 mg once daily. For plasma concentrations exceeding the mean Cmax level, e.g. 30 μm, a QTcF effect of 3.7 ms (90% CI −0.1, 7.5) can be predicted using the same model. The upper bound of the 90% CI of the projected ΔΔQTcF effect was below 10 ms for all plasma concentrations observed in both the SAD and the MAD part of the study (Figure 3A, B). In R6/2 mice treated with Selisistat (10 mg/kg, 30 mg/kg) for 6 weeks, no significant changes in body weight, food intake, or clinical signs of toxicity (lethargy, ataxia) were observed. Liver/kidney histology showed no abnormalities [1] - In Zucker fatty rats treated with Selisistat (15 mg/kg) for 8 weeks, serum creatinine and urea nitrogen (kidney function markers) were unchanged vs. vehicle, and no histopathological damage was observed in heart, spleen, or pancreas [3] - In healthy human volunteers, Selisistat (up to 60 mg) was well-tolerated; adverse events were mild (headache, nausea) and occurred in <10% of subjects. No significant changes in serum ALT, AST, creatinine, or QT interval (ECG) were observed [4] - Plasma protein binding of Selisistat in human plasma was 95%, determined via equilibrium dialysis [4] |
| 参考文献 |
|
| 其他信息 |
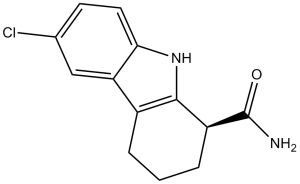
6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide is a member of the class of carbazoles that is 2,3,4,9-tetrahydro-1H-carbazole which is substituted at position 1 by an aminocarbohyl group and at position 6 by a chlorine. It is a member of carbazoles, a monocarboxylic acid amide and an organochlorine compound.
Selective inhibitor of SIRT1 that does not inhibit histone deacetylase (HDAC) or other sirtuin deacetylase family members (IC50 values are 98, 19600, 48700, > 100000 and > 100000 nM for SIRT1, SIRT2, SIRT3, HDAC and NADase respectively). Enhances p53 acetylation in response to DNA damaging agents. High-throughput screening against the human sirtuin SIRT1 led to the discovery of a series of indoles as potent inhibitors that are selective for SIRT1 over other deacetylases and NAD-processing enzymes. The most potent compounds described herein inhibit SIRT1 with IC50 values of 60-100 nM, representing a 500-fold improvement over previously reported SIRT inhibitors. Preparation of enantiomerically pure indole derivatives allowed for their characterization in vitro and in vivo. Kinetic analyses suggest that these inhibitors bind after the release of nicotinamide from the enzyme and prevent the release of deacetylated peptide and O-acetyl-ADP-ribose, the products of enzyme-catalyzed deacetylation. These SIRT1 inhibitors are low molecular weight, cell-permeable, orally bioavailable, and metabolically stable. These compounds provide chemical tools to study the biology of SIRT1 and to explore therapeutic uses for SIRT1 inhibitors.[2] Sirtuin (SIRT) is known to prevent nonalcoholic fatty liver disease (NAFLD); however, the role of SIRT4 in the progression of hepatic fibrosis remains unknown. We hypothesize that EX-527, a selective SIRT1 inhibitor, can inhibit the progression of high-fat diet (HFD)-induced hepatic fibrosis. We found that SIRT4 expression in the liver of NAFLD patients is significantly lower than that in normal subjects. In this study, EX-527 (5 µg/kg), administered to HFD rats twice a week for ten weeks, reduced the serum levels of triglyceride (TG), total cholesterol, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) and attenuated hepatic fibrosis evidenced by Masson's trichrome and hepatic fat by oil red-O staining. EX-527 upregulated SIRT2, SIRT3, and SIRT4 expression in the liver of HFD fed rats but downregulated transforming growth factor-β1 (TGF-β1) and α-smooth muscle actin (α-SMA) expression. It decreased proinflammatory cytokine production and hydroxyproline levels in the serum and SMAD4 expression and restored apoptotic protein (Bcl-2, Bax, and cleaved caspase-3) expression. These data propose a critical role for the SIRT4/SMAD4 axis in hepatic fibrogenesis. SIRT4 upregulation has the potential to counter HFD-induced lipid accumulation, inflammation, and fibrogenesis. We demonstrate that EX-527 is a promising candidate in inhibiting the progression of HFD-induced liver fibrosis.[3] Aim: Selisistat (SEN0014196), a first-in-class SirT1 inhibitor, is being developed as a disease-modifying therapy for Huntington's disease. This first-in-human study investigated the safety, pharmacokinetics and pharmacogenomics of single and multiple doses of selisistat in healthy male and female subjects. Method: In this double-blind, randomized, placebo-controlled study, seven cohorts of eight subjects received a single dose of selisistat at dose levels of 5, 25, 75, 150, 300 and 600 mg and four cohorts of eight subjects were administered 100, 200 and 300 mg once daily for 7 days. Blood sampling and safety assessments were conducted throughout the study. Results: Selisistat was rapidly absorbed and systemic exposure increased in proportion to dose in the 5-300 mg range. Steady-state plasma concentrations were achieved within 4 days of repeated dosing. The incidence of drug related adverse events showed no correlation with dose level or number of doses received and was comparable with the placebo group. No serious adverse events were reported and no subjects were withdrawn due to adverse events. There were no trends in clinical laboratory parameters or vital signs. No trends in heart rate or ECG parameters, including the QTc interval and T-wave morphology, were observed. There were no findings in physical or neurological examinations or postural control. Transcriptional alteration was observed in peripheral blood. Conclusion: Selisistat was safe and well tolerated by healthy male and female subjects after single doses up to 600 mg and multiple doses up to 300 mg day(-1).[4] Selisistat (SEN0014196; EX 527) is a potent, selective SIRT1 inhibitor, initially developed to target neurodegenerative diseases (e.g., Huntington’s disease) by reducing mutant huntingtin (mHtt) aggregation and neuroinflammation [1][2] - In hepatic steatosis and fibrosis, Selisistat exerts therapeutic effects by inhibiting SIRT1 to upregulate SIRT4, which reduces hepatic lipid accumulation and suppresses hepatic stellate cell activation [3] - Selisistat showed favorable pharmacokinetics in healthy humans (dose-proportional exposure, long half-life) and no significant toxicity at doses up to 60 mg, supporting its potential for clinical development [4] - The high selectivity of Selisistat for SIRT1 over other sirtuins and HDACs minimizes off-target effects, a key advantage for its use in chronic diseases (e.g., neurodegeneration, metabolic disorders) [2] |
| 分子式 |
C13H13CLN2O
|
|---|---|
| 分子量 |
248.7081
|
| 精确质量 |
248.071
|
| 元素分析 |
C, 62.78; H, 5.27; Cl, 14.25; N, 11.26; O, 6.43
|
| CAS号 |
49843-98-3
|
| 相关CAS号 |
(S)-Selisistat;848193-68-0;(R)-Selisistat;848193-69-1
|
| PubChem CID |
5113032
|
| 外观&性状 |
Off-white to light yellow solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
531.7±38.0 °C at 760 mmHg
|
| 熔点 |
179.0 to 183.0 °C
|
| 闪点 |
275.4±26.8 °C
|
| 蒸汽压 |
0.0±1.4 mmHg at 25°C
|
| 折射率 |
1.688
|
| LogP |
2.5
|
| tPSA |
58.9
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
17
|
| 分子复杂度/Complexity |
323
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1C([H])=C([H])C2=C(C=1[H])C1C([H])([H])C([H])([H])C([H])([H])C([H])(C(N([H])[H])=O)C=1N2[H]
|
| InChi Key |
FUZYTVDVLBBXDL-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C13H13ClN2O/c14-7-4-5-11-10(6-7)8-2-1-3-9(13(15)17)12(8)16-11/h4-6,9,16H,1-3H2,(H2,15,17)
|
| 化学名 |
6-Chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide
|
| 别名 |
Selisistat; EX 527; SEN 0014196; 6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide; SEN0014196; SIRT1 Inhibitor III; EX527; SEN-0014196; SEN0014196; EX-527
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (10.05 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (10.05 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (10.05 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 1% DMSO+30% polyethylene glycol+1% Tween 80:14mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.0207 mL | 20.1037 mL | 40.2075 mL | |
| 5 mM | 0.8041 mL | 4.0207 mL | 8.0415 mL | |
| 10 mM | 0.4021 mL | 2.0104 mL | 4.0207 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04184323 | Withdrawn | Drug: EX-527 (Selisistat) Drug: Placebo |
Endometriosis Uterine Diseases |
Wake Forest University Health Sciences | January 2022 | Phase 2 |
Pharmacologic blockade of SIRT1 blunts the orexigenic action of ghrelin.Diabetes.2011 Apr;60(4):1177-85. |
Mice lacking p53 do not respond to ghrelin injection.Diabetes.2011 Apr;60(4):1177-85. |
Pharmacologic blockade of SIRT1 does not modify the ghrelin-induced GH secretion.Diabetes.2011 Apr;60(4):1177-85. |