| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg | |||
| 500mg | |||
| Other Sizes |
| 靶点 |
glucagon-like peptide-1 receptor ( IC50 = 3.22 nM )
|
|---|---|
| 体外研究 (In Vitro) |
在人脐静脉内皮细胞中,exendin-4 以剂量依赖性方式显着增加 NO 产生、内皮 NO 合酶 (eNOS) 磷酸化和 GTP 环水解酶 1 (GTPCH1) 水平[2]。 Exendin-4 对 MCF-7 乳腺癌细胞显示出细胞毒性作用,48 小时时 IC50 为 5 μM[3]。
Exenatide (Exendin-4)是一种合成形式的GLP-1类似物,用于治疗2型糖尿病。由于患有糖尿病的女性乳腺癌症发病率和死亡率较高,我们研究了肠促胰岛素药物Exenatide (Exendin-4)对癌症乳腺癌细胞的影响。本研究旨在探讨Exenatide (Exendin-4)对MCF-7乳腺癌症细胞的抗癌作用机制。通过XTT法测定Exenatide (Exendin-4)的细胞毒性作用。在48小时时检测到MCF-7细胞的IC50剂量为5μM。通过实时PCR评估基因信使RNA(mRNA)的表达。根据结果,与对照组细胞相比,剂量组细胞中caspase-9、Akt和MMP2的表达降低。与对照组细胞相比,剂量组细胞中p53、caspase-3、caspase-8、caspase-10、BID、DR4、DR5、FADD、TRADD、PARP、PTEN、PUMA、NOXA、APAF、TIMP1和TIMP2的表达增加。分别通过基质胶室、集落形成试验和伤口愈合试验检测Exenatide (Exendin-4)对细胞侵袭、集落生成和细胞迁移的影响。综上所述,人们认为Exenatide (Exendin-4)通过影响MCF-7细胞的凋亡、侵袭、迁移和集落形成来显示抗癌活性。Exenatide (Exendin-4)可以是用于治疗乳腺癌症的治疗剂,作为单独的或与其他药物组合。由于癌症的分子生物学涉及一个复杂的相互连接的信号通路网络,在细胞生长、存活和细胞侵袭中发挥作用,因此需要更详细的研究来确定GLP-1对乳腺癌症细胞的作用途径[3]. |
| 体内研究 (In Vivo) |
与对照组相比,低剂量和高剂量 exendin-4 治疗 ob/ob 小鼠均可改善血清 ALT 并降低血糖,并计算出 HOMA 评分。 Exendin-4 治疗的 ob/ob 小鼠在研究期的最后 4 周内净体重增加显着减少[4]。用exendin-4治疗的动物比对照大鼠有更多的胰腺腺泡炎症、更多的固缩核并且体重显着减轻。 Exendin-4 治疗与大鼠体内瘦素水平降低以及 HOMA 值降低相关[5]。艾塞那肽引起大鼠胸主动脉的剂量依赖性松弛,这是通过 GLP-1 受体引起的,主要由 H2S 介导,也由 NO 和 CO 介导[6]。
非酒精性脂肪肝(NAFLD)是肝病学中一个新兴的问题,与胰岛素抵抗有关。Exendin-4是胰高血糖素样肽(GLP)受体的肽激动剂,可促进胰岛素分泌。本研究旨在确定Exendin-4的给药是否会逆转ob/ob小鼠的肝脂肪变性。Ob/Ob小鼠或其瘦同窝小鼠用Exendin-4[10微克/千克或20微克/千克]治疗60天。收集血清以测量胰岛素、脂联素、空腹血糖、血脂和转氨酶浓度。采集肝组织进行组织学检查、实时RT-PCR分析和氧化应激测定。分离大鼠肝细胞并用GLP-1处理。Ob/Ob小鼠在Exendin-4治疗期间增加的净重持续减少。Exendin-4治疗的ob/ob小鼠的血糖和肝脂肪变性显著降低。根据稳态模型评估计算,Exendin-4改善了ob/ob小鼠的胰岛素敏感性。在用Exendin-4治疗的ob/ob小鼠中,硫代巴比妥反应物质作为氧化应激标志物的测量值显著降低。最后,GLP-1处理的肝细胞导致cAMP产生显著增加,硬脂酰辅酶a去饱和酶1和与脂肪酸合成相关的基因的mRNA表达减少;与脂肪酸氧化相关的基因则相反。总之,Exendin-4似乎通过提高胰岛素敏感性有效逆转肥胖/肥胖小鼠的肝脂肪变性。我们的数据表明,肝脏中的GLP-1蛋白对肝细胞脂肪代谢有新的直接影响。[4] 目的/假设:Exendin-4是胰高血糖素样肽受体的39个氨基酸的激动剂,已被批准用于治疗2型糖尿病。许多报告描述了用毒蜥外泌肽-4(艾塞那肽)治疗的人急性胰腺炎发病率的增加。之前的研究已经评估了毒蜥外泌肽-4对β细胞和β细胞功能的影响。我们评估了毒蜥外泌肽-4对大鼠胰腺的组织学和生化影响。 方法:我们研究了20只Sprague-Dawley雄性大鼠,其中10只用毒蜥外泌肽-4治疗,10只作为对照。研究期为75天。取出血清和胰腺组织进行生化和组织学研究。比较两组的血糖、淀粉酶、脂肪酶、胰岛素和脂肪细胞因子。 结果:与对照组大鼠相比,用毒蜥外泌肽-4治疗的动物胰腺腺泡炎症更多,核固缩更多,体重明显减轻。它们的血清脂肪酶水平也高于对照组动物。Exendin-4治疗与未治疗的对照组相比,胰岛素和瘦素水平较低,HOMA值也较低。 结论/解释:尽管与对照组动物相比,在大鼠中使用毒蜥外泌肽-4与体重增加减少、胰岛素抵抗降低和瘦素水平降低有关,但在大鼠体内长期使用毒蜥内泌肽-4会导致胰腺腺泡炎症和固缩。这引发了人们对接受肠促胰岛素模拟疗法的人诱发急性胰腺炎的可能性的重要担忧。[5] 背景:据报道,GLP-1激动剂艾塞那肽(exendin-4)可以降低血压。毒蜥外泌肽-4的剂量依赖性血管舒张作用已被证实,尽管其确切机制尚未完全描述。在这里,我们的目的是为艾塞那肽可能降低中枢(主动脉)血压的假设提供体外证据,这涉及三种气体递质,即一氧化氮(NO)、一氧化碳(CO)和硫化氢(H2S)。 方法:我们测定了艾塞那肽对成年大鼠离体胸主动脉环的血管活性作用。将两毫米长的血管段放置在钢丝肌描记图中,并与产生三种气体传递子的酶的抑制剂、活性氧形成抑制剂、前列腺素合成抑制剂、蛋白激酶抑制剂、钾通道抑制剂或Na+/Ca2+-交换器抑制剂预孵育。 结果:艾塞那肽可引起大鼠胸主动脉剂量依赖性舒张,这是通过GLP-1受体诱发的,主要由H2S介导,也由NO和CO介导。前列腺素和超氧自由基也在舒张中起作用。可溶性鸟苷酸环化酶的抑制显著降低了血管舒张作用。我们发现ATP敏感、电压门控和钙激活的大电导钾通道也参与血管舒张,但似乎KCNQ型电压门控钾通道的抑制导致了血管舒张速率的显著降低。抑制Na+/Ca2+-交换器可消除大部分血管舒张。 结论:艾塞那肽诱导大鼠胸主动脉血管舒张,三种气体递质都有作用。我们为艾塞那肽降低中心(主动脉)血压的潜在能力提供了体外证据,这可能具有相关的临床意义[6]。 |
| 酶活实验 |
完整细胞中肽与GLP-1受体的竞争性结合[1]
如Montrose Rafizadeh等人所述进行结合研究。简而言之,CHO/GLP-1R细胞在12孔板上生长至融合,并在实验前用无血清火腿F-12培养基洗涤2小时。在0.5 ml结合缓冲液中洗涤两次后,细胞在4°C下与0.5 ml缓冲液一起孵育过夜,缓冲液含有2%牛血清白蛋白、50μm DPP-IV抑制剂、400个激肽释放酶失活单位(KIU)抑肽酶、10 mM葡萄糖、1-1000 nM GLP-1或其他肽和30000 cpm[125I]GLP-1。孵育结束时,丢弃上清液,用冰冷的磷酸盐缓冲盐水(PBS)洗涤细胞三次,在室温下用0.5 ml 0.5 M NaOH和0.1%十二烷基硫酸钠孵育10分钟。在ICN-Apec Seriesγ计数器中测量细胞裂解物的放射性。特异性结合被确定为总结合减去与在大量过量未标记的GLP-1(1μM)存在下孵育的细胞相关的放射性。 Exendin-4是一种39个氨基酸(AA)的肽,是胰高血糖素样肽-1(GLP-1)受体的长效激动剂。因此,作为2型糖尿病的长期治疗,它可能比GLP-1更可取。Exendin-4(Ex-4)与GLP-1不同,不被二肽基肽酶IV(DPP IV)降解,不易被中性内肽酶降解,并且具有GLP-1中缺失的九个AA C末端序列。在这里,我们研究了这九种氨基酸对Ex-4、截短的Ex-4类似物序列以及添加了全部或部分C末端序列的天然GLP-1和GLP-1类似物的生物活性的重要性。我们发现,从Ex-4中去除这些AA以产生Ex(1-30)降低了与Ex-4相比对GLP-1受体(GLP-1R)的亲和力(IC50:Ex-4,3.22+/-0.9 nM;Ex(1-30),32+/-5.8 nM),但使其与GLP-1的亲和力相当(IC50:44.9+/-3.2 nM)。将这九个AA序列添加到GLP-1中提高了GLP-1和DPP IV抗性类似物GLP-1 8-甘氨酸对GLP-1受体的亲和力(IC50:GLP-1 Gly8[GG],220+/-23nM;GLP-1 Gly8-Ex(31-39),74+/-11nM)。胰岛素瘤细胞系中cAMP反应的观察显示了类似的生物活性趋势[1]。 |
| 细胞实验 |
细胞毒性试验[3]
按照制造商的说明,使用台盼蓝染料排除试验和XTT试验进行MCF-7细胞中艾塞那肽(Exendin-4)的细胞毒性试验和IC50剂量测定。 伤口愈合试验[3] 对照组和剂量组细胞以每孔106个细胞的方式接种在60×15 mm的细胞培养皿中,并在37°C和5%CO2的条件下生长过夜。使用无菌200μl塑料移液管尖端在细胞融合单层上做直线划痕后,用5μM艾塞那肽(Exendin-4)处理80%融合的对照组和剂量组细胞。为了去除碎屑并使划痕边缘光滑,用2ml无血清DMEM洗涤细胞。在划痕后0、16、24和48小时拍摄MCF-7细胞增殖的图像。划痕试验一式三份。 |
| 动物实验 |
Rats: 20 Sprague-Dawley male rats, ten of which are treated with exendin-4 (10 μg/kg) and ten of which are used as controls. There are 75 days in the study period. Pancreatic tissue and serum are extracted for histological and biochemical analysis. The two groups' levels of blood glucose, lipase, amylase, and adipocytokines are compared[5].
Mice: For the first 14 days, 10 μg/kg is administered to the exendin-4 treatment groups every 24 hours. This therapy is the initiating stage. Every 24 hours, the corresponding control mice (lean and ob/ob) are given saline. Exendin-4-treated mice are split into two groups at random after 14 days: the first group is given a high dose of exendin-4 (20 μg/kg) every 12 hours, while the second group is given a low dose of exendin-4 (10 μg/kg) every 12 hours. Every twelve hours, saline is still given to the control mice. Every day for the duration of the 60-day treatment, the mice are weighed[4]. Use of ob/ob Mouse Model and Treatment With Exenatide (Exendin-4) [4] Obese male (ob/ob) 6-week-old mice and their lean littermates were used. For both ob/ob mice and their lean littermates the we followed the same treatment strategy. All animals were treated for 60 days. The Exenatide (Exendin-4) treatment groups were treated with 10 μg/kg every 24 hours for the first 14 days. This treatment was the induction phase. Respective control mice (lean and ob/ob) received saline every 24 hours. After 14 days Exendin-4–treated mice were randomly divided into two groups: one group received high dose Exendin-4 (20 μg/kg) every 12 hours, while the second group continued with low dose Exendin-4 (10 μg/kg) every 12 hours. Exenatide (Exendin-4) administration and tissue removal [5] Highly purified drug (Exenatide (Exendin-4)) was stored at −70°C and dosages prepared as needed. In line with previous publications on exendin-4 in rats and in order to better elucidate the effect of exendin-4 on the pancreas, it was decided to use a dose of 10 μg/kg [7]. This dosage was administered subcutaneously to the treated group each day immediately before the 12 h dark cycle when rats are known to feed. Animal weights were recorded weekly and doses adjusted according to weight. The ten exendin-treated rats and ten control animals were killed after 75 days of treatment. Serum was obtained from each animal and necropsy tissue collection specimens were fixed in 10% formalin. Vasoreactivity experiments [6] After all vessel segments had reached a stable contraction plateau, increasing doses of Exenatide (Exendin-4) were administered to the organ baths, and relaxant responses were assessed. The dose of exenatide that was applied to relax the aorta correlated with the dose of epinephrine we used to preconstrict the vessels. Plasma epinephrine level is approximately 30 pM at rest, while in our experiments we used 100 nM, which is a 3000 times higher concentration. The plasma exenatide level was found to be 70 pM, while in our experiments we used a 4500 times higher concentration. In order to identify the extracellular and intracellular mediators of the vasodilator effect of Exenatide (Exendin-4) we performed a series of experiments. Prior to contracting the vessels with epinephrine we preincubated the vessels (n = 5 of each experiment) with different materials. To determine whether the vasodilation due to exenatide evoked via the GLP-1R, we preincubated vessels with GLP-1R antagonist exendin(9–39) (32 μM, 30 min). Because the affinity of exendin(9–39) to bind GLP-1R is smaller than that of exenatide, we applied a ten times higher concentration of the receptor antagonist than the highest dose of exenatide. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Exenatide reaches a peak plasma concentration in 2.1 hours. Because exenatide is administerd subcutaneously, the bioavailability is 1. Exenatide is mainly eliminated by glomerular filtration followed by proteolysis before finally being eliminated in the urine. 28.3L. 9.1 L/hour. Following a single dose of Bydureon, exenatide is released from the microspheres over approximately 10 weeks. There is an initial period of release of surface-bound exenatide followed by a gradual release of exenatide from the microspheres, which results in two subsequent peaks of exenatide in plasma at around week 2 and week 6 to 7, respectively, representing the hydration and erosion of the microspheres. Following initiation of once every 7 days (weekly) administration of 2 mg Bydureon, gradual increase in the plasma exenatide concentration is observed over 6 to 7 weeks. After 6 to 7 weeks, mean exenatide concentrations of approximately 300 pg/mL were maintained over once every 7 days (weekly) dosing intervals indicating that steady state was achieved. Nonclinical studies have shown that exenatide is predominantly eliminated by glomerular filtration with subsequent proteolytic degradation. The mean apparent clearance of exenatide in humans is 9.1 L/hr and the mean terminal half-life is 2.4 hr. These pharmacokinetic characteristics of exenatide are independent of the dose. In most individuals, exenatide concentrations are measurable for approximately 10 hr post-dose. The mean apparent volume of distribution of exenatide following SC administration of a single dose of Byetta is 28.3 L. Following SC administration to patients with type 2 diabetes, exenatide reaches median peak plasma concentrations in 2.1 hr. The mean peak exenatide concentration (Cmax) was 211 pg/mL and overall mean area under the time-concentration curve (AUC0-inf) was 1036 pg hr/mL following SC administration of a 10 ug dose of Byetta. Exenatide exposure (AUC) increased proportionally over the therapeutic dose range of 5 ug to 10 ug. The Cmax values increased less than proportionally over the same range. Similar exposure is achieved with SC administration of Byetta in the abdomen, thigh, or upper arm. For more Absorption, Distribution and Excretion (Complete) data for Exenatide (6 total), please visit the HSDB record page. Metabolism / Metabolites Exenatide is filtered through the glomerulus before being degraded to smaller peptides and amino acids by dipeptidyl peptidase-4, metalloproteases, endopeptidase 24-11, amino proteases, and serine proteases. It is currently believed that the metalloproteases are responsible for most of the degradation of exenatide. Exenatide is metabolised to small peptides <3 amino acids in length by enzymes in the kidney. Biological Half-Life 2.4 hours Terminal half life varied from 18 minutes in mice up to 114 minutes in rats. Mean terminal half-life /in humans/ is 2.4 hr |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Exenatide is a white to off-white powder formulated into solution for subcutaneous use. Exenatide is available in both a twice daily formulation and an extended-release formulation that is administered weekly. Exenatide is a synthetic, long-acting human glucagon-like peptide-1 (GLP-1) receptor agonist (incretin mimetic). It is used as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. HUMAN EXPOSURE AND TOXICITY: Overdose of exenatide has been reported in a clinical study. Effects have included severe nausea, severe vomiting, and rapidly declining blood glucose concentrations. Post marketing reports also include acute pancreatitis, including fatal and nonfatal hemorrhagic or necrotizing pancreatitis requiring hospitalization and serious hypersensitivity reactions (e.g. anaphylaxis and angioedema). Deterioration of renal function (e.g., increased serum creatinine concentrations, renal impairment/insufficiency, and chronic renal failure, acute renal failure sometimes requiring hemodialysis or kidney transplantation) has also been reported with the use of exenatide. Exenatide extended-release also caused thyroid C-cell tumors at clinically relevant exposures in rats. It is unknown whether the drug causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans, as human relevance could not be determined by clinical or nonclinical studies. Therefore, exenatide extended release is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2. ANIMAL STUDIES: Exenatide had no adverse effects on fertility when given to male mice at doses up to 760 ug/kg/day. However, exenatide did cause developmental toxicity in rats, mice and rabbits. Fetuses from pregnant rats given subcutaneous doses of exenatide extended-release at 0.3, 1, or 3 mg/kg on gestation days 6, 9, 12, and 15 demonstrated reduced fetal growth at all doses and produced skeletal ossification deficits at 1 and 3 mg/kg in association with maternal effects (decreased food intake and decreased body weight gain). In pregnant mice given sc doses of 6, 68, 460, or 760 ug/kg/day from gestation day 6 through 15 (organogenesis), cleft palate (some with holes) and irregular fetal skeletal ossification of rib and skull bones were observed at 6 ug/kg/day. In pregnant rabbits given sc doses of 0.2, 2, 22, 156, or 260 ug/kg/day from gestation day 6 through 18 (organogenesis), irregular fetal skeletal ossifications were observed at 2 ug/kg/day. Studies for the carcinogenicity potential of exenatide were also conducted in rats. Benign thyroid C-adenomas were observed in female rats given extenatide by sc injection at doses of 18, 70, or 250 ug/kg/day. In another carcinogenicity study with exenatide extended-release male and female rats were administrated doses of 0.3, 1.0, and 3.0 mg/kg by subcutaneous injection every other week. A statistically significant increase in thyroid C-cell tumor incidence was observed in both males and females. The incidence of C-cell adenomas was statistically significantly increased at all doses (27%-31%) in females and at 1.0 and 3.0 mg/kg (46% and 47%, respectively) in males compared with the control group (13% for males and 7% for females). A statistically significantly higher incidence of C-cell carcinomas occurred in the high-dose group females (6%), while numerically higher incidences of 3%, 7%, and 4% (nonstatistically significant versus controls) were noted in the low-, mid-, and high-dose group males compared with the control group (0% for both males and females). An increase in benign fibromas was seen in the skin subcutis at injection sites of males given 3 mg/kg. No treatment-related injection-site fibrosarcomas were observed at any dose. Exenatide was not mutagenic or clastogenic, with or without metabolic activation, in the Ames bacterial mutagenicity assay or chromosomal aberration assay in Chinese hamster ovary cells. Exenatide was negative in the in vivo mouse micronucleus assay Hepatotoxicity Liver injury due to exenatide must be rare, if it occurs at all. In large clinical trials, serum enzyme elevations were no more common with exenatide therapy than with placebo or comparator agents, and no instances of clinically apparent liver injury were reported. Since licensure, there have been no published case reports of hepatotoxicity due to exenatide and the product label does not list liver injury as an adverse event. Exenatide has been linked to rare instances of acute pancreatitis, but even this complication is usually not associated with elevations in serum bilirubin and aminotransferase levels. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of exenatide during breastfeeding. Because exenatide is a large peptide molecule with a molecular weight of 4187 daltons, the amount in milk is likely to be very low and absorption is unlikely because it is probably partially destroyed in the infant's gastrointestinal tract. It has a short half-life, which might make it a better choice among drugs in this class for nursing mothers. If exenatide is required by the mother, it is not a reason to discontinue breastfeeding. However, until more data become available, exenatide should be used with caution during breastfeeding, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Protein binding of exenatide has not been determined. Interactions Increases in international normalized ratio (INR), sometimes associated with bleeding, have been reported during postmarketing experience with concomitant use of exenatide and warfarin. In a drug interaction study, no clinically important changes in warfarin (S- or R-enantiomer) AUC, peak plasma concentrations, or therapeutic response (as indicated by INR) were observed when warfarin sodium (single 25-mg dose) was administered 35 minutes after exenatide (5 ug subcutaneously twice daily for 2 days, then 10 ug twice daily for 7 days); however, the time to peak warfarin concentration was delayed by approximately 2 hours. In patients receiving warfarin, prothrombin time should be monitored more frequently after initiating or altering exenatide therapy; once a stable prothrombin time has been achieved, prothrombin times may be monitored at intervals usually recommended for patients receiving warfarin therapy. In healthy women, repeated daily administration of a fixed-combination oral contraceptive (30 ug of ethinyl estradiol and 150 ug of levonorgestrel) 30 minutes after subcutaneous injection of exenatide (10 ug twice daily) decreased the peak plasma concentrations of ethinyl estradiol and levonorgestrel by 45 and 27%, respectively, and delayed the time to peak plasma concentrations of ethinyl estradiol and levonorgestrel by 3 and 3.5 hours, respectively. Repeated daily administration of the fixed-combination oral contraceptive 1 hour prior to administration of exenatide decreased the mean peak plasma concentration of ethinyl estradiol by 15%; however, the mean peak plasma concentration of levonorgestrel was not substantially changed. Exenatide did not alter the mean trough concentrations of levonorgestrel following repeated daily administration of the fixed-combination oral contraceptive for both regimens; however, the mean trough concentration of ethinyl estradiol increased by 20% when the fixed-combination oral contraceptive was administered 30 minutes after exenatide injection. In this study, the effect of exenatide on the pharmacokinetics of oral contraceptives was confounded by the possible effect of food on oral contraceptives. Therefore, oral contraceptives should be administered at least 1 hour prior to exenatide administration. Administration of exenatide (10 ug subcutaneously twice daily) 30 minutes before lovastatin (single 40-mg oral dose) decreased the lovastatin AUC and peak plasma concentration by approximately 40 and 28%, respectively, and delayed the time to peak plasma concentration of lovastatin by 4 hours. In clinical trials, the use of exenatide in patients already receiving HMG-CoA reductase inhibitors (statins) was not associated with consistent changes in lipid profiles compared to baseline. In patients with mild to moderate hypertension receiving stable dosages of lisinopril (5-20 mg daily), exenatide (10 ug subcutaneously twice daily) did not alter the steady-state AUC or peak plasma concentration of lisinopril or the 24-hour mean systolic and diastolic blood pressure. However, the steady-state time to peak plasma concentration of lisinopril was delayed by 2 hours. For more Interactions (Complete) data for Exenatide (10 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Hypoglycemic Agents Byetta is a glucagon-like peptide-1 (GLP-1) receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus /Included in US product label/ Bydureon is a glucagon-like peptide-1 (GLP-1) receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. Bydureon is an extended-release formulation of exenatide. Do not coadminister with Byetta. /Included in US product label/ Prior treatment with Byetta is not required when initiating Bydureon therapy. If the decision is made to start Bydureon in an appropriate patient already taking Byetta, Byetta should be discontinued. Patients changing from Byetta to Bydureon may experience transient (approximately 2 weeks) elevations in blood glucose concentrations. For more Therapeutic Uses (Complete) data for Exenatide (8 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ WARNING: RISK OF THYROID C-CELL TUMORS. Exenatide extended-release causes thyroid C-cell tumors at clinically relevant exposures in rats. It is unknown whether Bydureon causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans, as human relevance could not be determined by clinical or nonclinical studies. Bydureon is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2. Acute pancreatitis, including fatal and nonfatal hemorrhagic or necrotizing pancreatitis requiring hospitalization, has been reported during postmarketing experience with exenatide. Persistent, severe abdominal pain, which may be accompanied by vomiting, is the hallmark symptom of acute pancreatitis. Most patients who have developed pancreatitis have had at least one other risk factor for acute pancreatitis (e.g., gallstones, severe hypertriglyceridemia, alcohol use) and have required hospitalization. Some patients have developed serious complications including dehydration and renal failure, suspected ileus, phlegmon, and ascites. Acute or worsening pancreatitis has been associated temporally with an increase in exenatide dosage from 5 ug to 10 ug twice daily, the maximum recommended dosage, in some patients. Symptoms of acute pancreatitis (e.g., nausea, vomiting, abdominal pain) recurred upon rechallenge with the drug in several patients; abdominal pain abated after permanent discontinuance of the drug in one patient. Most patients have improved upon discontinuance of exenatide. The US Food and Drug Administration (FDA) is evaluating unpublished findings suggesting an increased risk of pancreatitis and precancerous cellular changes (pancreatic duct metaplasia) in patients with type 2 diabetes mellitus receiving incretin mimetics (exenatide, liraglutide, sitagliptin, saxagliptin, alogliptin, or linagliptin). These findings are based on examination of a small number of pancreatic tissue specimens taken from patients who died from unspecified causes while receiving an incretin mimetic. FDA has not yet reached any new conclusions about safety risks with incretin mimetics. FDA will notify healthcare professionals of its conclusions and recommendations when the review is complete, or when the agency has additional information to report. FDA states that at this time clinicians should continue to follow the recommendations in the prescribing information for incretin mimetics. The manufacturer states that after initiation of exenatide, and after increases in dosage, patients should be observed carefully for signs and symptoms of acute pancreatitis (e.g., unexplained, persistent severe abdominal pain that may radiate to the back; nausea; vomiting; elevated serum amylase or lipase concentrations). If pancreatitis is suspected, therapy with exenatide and other potentially suspect drugs should be promptly discontinued, confirmatory tests performed (e.g., serum amylase or lipase concentrations, radiologic imaging), and appropriate therapy initiated. Exenatide should not be resumed if pancreatitis is confirmed. Exenatide has not been studied in patients with a history of pancreatitis; other antidiabetic therapies should be considered in such patients. Deterioration of renal function (e.g., increased serum creatinine concentrations, renal impairment/insufficiency, worsened chronic renal failure, acute renal failure sometimes requiring hemodialysis or kidney transplantation) has been reported rarely with exenatide. Some of these events occurred in patients experiencing nausea, vomiting, and/or diarrhea with or without dehydration; these adverse effects may have contributed to development of altered renal function in these patients. Some of these events also occurred in patients receiving exenatide in combination with other agents known to affect renal function or hydration status (e.g., angiotensin-converting enzyme inhibitors, nonsteroidal anti-inflammatory agents, diuretics). Exenatide has not been found to be directly nephrotoxic in preclinical or clinical studies. Renal effects usually have been reversible with supportive treatment and discontinuance of potentially causative agents, including exenatide. Altered renal function may be a consequence of diabetes mellitus, independent of any risk associated with exenatide therapy. Clinicians should closely monitor patients receiving exenatide for signs and symptoms of renal dysfunction and consider discontinuance of the drug if renal dysfunction is suspected and cannot be explained by other causes. For more Drug Warnings (Complete) data for Exenatide (15 total), please visit the HSDB record page. Pharmacodynamics When patients take exenatide the body's natural response to glucose is modulated. More insulin and less glucagon are released in response to glucose, though in cases of hypoglycemia a normal amount of glucagon is released. Exenatide also slows gastric emptying, leading to a slower and prolonged release of glucose into the systemic circulation. Together these effects prevent hyper and hypoglycemia. Glucagon-like peptide-1 (GLP-1) may have direct favorable effects on cardiovascular system. The aim of this study was to investigate the effects of the GLP-1 analog exenatide on improving coronary endothelial function in patients with type 2 diabetes and to investigate the underlying mechanisms. The newly diagnosed type 2 diabetic subjects were enrolled and given either lifestyle intervention or lifestyle intervention plus exenatide treatment. After 12-wk treatment, coronary flow velocity reserve (CFVR), an important indicator of coronary endothelial function, was improved significantly, and serum levels of soluble intercellular adhesion molecule-1 (sICAM-1) and soluble vascular cell adhesion molecule-1 (sVCAM-1) were remarkably decreased in the exenatide treatment group compared with the baseline and the control group. Notably, CFVR was correlated inversely with hemoglobin A1c (Hb A1c) and positively with high-density lipoprotein cholesterol (HDL-C). In human umbilical vein endothelial cells, exendin-4 (a form of exenatide) significantly increased NO production, endothelial NO synthase (eNOS) phosphorylation, and GTP cyclohydrolase 1 (GTPCH1) level in a dose-dependent manner. The GLP-1 receptor (GLP-1R) antagonist exendin (9-39) or GLP-1R siRNA, adenylyl cyclase inhibitor SQ-22536, AMPK inhibitor compound C, and PI3K inhibitor LY-294002 abolished the effects of exendin-4. Furthermore, exendin-4 reversed homocysteine-induced endothelial dysfunction by decreasing sICAM-1 and reactive oxygen species (ROS) levels and upregulating NO production and eNOS phosphorylation. Likewise, exendin (9-39) diminished the protective effects of exendin-4 on the homocysteine-induced endothelial dysfunction. In conclusion, exenatide significantly improves coronary endothelial function in patients with newly diagnosed type 2 diabetes. The effect may be mediated through activation of AMPK/PI3K-Akt/eNOS pathway via a GLP-1R/cAMP-dependent mechanism.[2] |
| 分子式 |
C₁₈₄H₂₈₂N₅₀O₆₀S
|
|---|---|
| 分子量 |
4186.57
|
| 精确质量 |
4184.03
|
| 元素分析 |
C, 52.79; H, 6.79; N, 16.73; O, 22.93; S, 0.77
|
| CAS号 |
141758-74-9
|
| 相关CAS号 |
Exendin-4 acetate; 914454-01-6
|
| PubChem CID |
53396299
|
| 序列 |
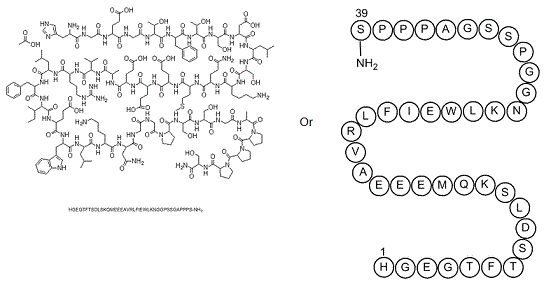
His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp-Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-NH2
|
| 短序列 |
HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPPS-NH2
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
-21
|
| tPSA |
1775.05
|
| 氢键供体(HBD)数目 |
58
|
| 氢键受体(HBA)数目 |
66
|
| 可旋转键数目(RBC) |
135
|
| 重原子数目 |
295
|
| 分子复杂度/Complexity |
10300
|
| 定义原子立体中心数目 |
0
|
| SMILES |
[HGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPPS-NH2]
|
| InChi Key |
HTQBXNHDCUEHJF-XWLPCZSASA-N
|
| InChi Code |
InChI=1S/C184H282N50O60S/c1-16-94(10)147(178(289)213-114(52-58-144(257)258)163(274)218-121(73-101-77-195-105-39-24-23-38-103(101)105)168(279)215-116(68-90(2)3)165(276)205-107(41-26-28-61-186)158(269)219-122(75-134(189)243)154(265)198-79-135(244)196-83-139(248)231-63-30-43-129(231)175(286)225-127(87-238)174(285)223-125(85-236)155(266)200-80-136(245)202-96(12)181(292)233-65-32-45-131(233)183(294)234-66-33-46-132(234)182(293)232-64-31-44-130(232)176(287)222-124(84-235)150(190)261)229-170(281)119(71-99-34-19-17-20-35-99)217-166(277)117(69-91(4)5)214-159(270)108(42-29-62-194-184(191)192)212-177(288)146(93(8)9)228-151(262)95(11)203-156(267)111(49-55-141(251)252)208-161(272)112(50-56-142(253)254)209-162(273)113(51-57-143(255)256)210-164(275)115(59-67-295-15)211-160(271)110(47-53-133(188)242)207-157(268)106(40-25-27-60-185)206-172(283)126(86-237)224-167(278)118(70-92(6)7)216-169(280)123(76-145(259)260)220-173(284)128(88-239)226-180(291)149(98(14)241)230-171(282)120(72-100-36-21-18-22-37-100)221-179(290)148(97(13)240)227-138(247)82-199-153(264)109(48-54-140(249)250)204-137(246)81-197-152(263)104(187)74-102-78-193-89-201-102/h17-24,34-39,77-78,89-98,104,106-132,146-149,195,235-241H,16,25-33,40-76,79-88,185-187H2,1-15H3,(H2,188,242)(H2,189,243)(H2,190,261)(H,193,201)(H,196,244)(H,197,263)(H,198,265)(H,199,264)(H,200,266)(H,202,245)(H,203,267)(H,204,246)(H,205,276)(H,206,283)(H,207,268)(H,208,272)(H,209,273)(H,210,275)(H,211,271)(H,212,288)(H,213,289)(H,214,270)(H,215,279)(H,216,280)(H,217,277)(H,218,274)(H,219,269)(H,220,284)(H,221,290)(H,222,287)(H,223,285)(H,224,278)(H,225,286)(H,226,291)(H,227,247)(H,228,262)(H,229,281)(H,230,282)(H,249,250)(H,251,252)(H,253,254)(H,255,256)(H,257,258)(H,259,260)(H4,191,192,194)/t94-,95-,96-,97+,98+,104-,106-,107-,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,146-,147-,148-,149-/m0/s1
|
| 化学名 |
(4S)-5-[[2-[[(2S,3R)-1-[[(2S)-1-[[(2S,3R)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-5-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S,3S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-6-amino-1-[[(2S)-4-amino-1-[[2-[[2-[(2S)-2-[[(2S)-1-[[(2S)-1-[[2-[[(2S)-1-[(2S)-2-[(2S)-2-[(2S)-2-[[(2S)-1-amino-3-hydroxy-1-oxopropan-2-yl]carbamoyl]pyrrolidine-1-carbonyl]pyrrolidine-1-carbonyl]pyrrolidin-1-yl]-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]carbamoyl]pyrrolidin-1-yl]-2-oxoethyl]amino]-2-oxoethyl]amino]-1,4-dioxobutan-2-yl]amino]-1-oxohexan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-1-oxopropan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-4-methylsulfanyl-1-oxobutan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-1-oxohexan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-carboxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-2-oxoethyl]amino]-4-[[2-[[(2S)-2-amino-3-(1H-imidazol-4-yl)propanoyl]amino]acetyl]amino]-5-oxopentanoic acid
|
| 别名 |
DA 3091; ITCA 650; LY 2148568; LY2148568; Byetta; Exenatide; Exendin-4; 141758-74-9; Exendin 4 (Heloderma suspectum); PT302; AC 2993; Exenatide; AC 2993A; AC-2993; Exendin-4; AC002993; AC2993; AC2993A; Bydureon
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮和光照。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O: ~33.3 mg/mL (~8.0 mM)
DMSO: ≥ 32 mg/mL (~7.6 mM) Ethanol: < 1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
Note: 如何溶解多肽产品?请参考本产品网页右上角“产品说明书”文件,第4页。 配方 1 中的溶解度: ≥ 2.5 mg/mL (0.60 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (0.60 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (0.60 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 100 mg/mL (23.89 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶 (<60°C). 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 0.2389 mL | 1.1943 mL | 2.3886 mL | |
| 5 mM | 0.0478 mL | 0.2389 mL | 0.4777 mL | |
| 10 mM | 0.0239 mL | 0.1194 mL | 0.2389 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Brain Activation and Satiety in Children 2
CTID: NCT04520490
Phase: Phase 3 Status: Active, not recruiting
Date: 2024-05-14
|
|
|
|
|
|