| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|

| 靶点 |
NPC1L1; Nrf2
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:依折麦布可显着降低总胆固醇、低密度脂蛋白胆固醇和甘油三酯,并小幅但显着地增加高密度脂蛋白胆固醇。依折麦布可减少 Caco-2 细胞中 31% 的胆固醇转运,但不会减少视黄醇的转运。依折麦布导致表面受体 SR-BI、Niemann-Pick 型 C1 Like 1 蛋白 (NPC1L1) 和 ATP 结合盒转运蛋白、亚家族 A (ABCA1) 和核受体视黄酸受体 (ABCA1) 的 mRNA 表达显着降低。通过 Caco-2 细胞中的实时 PCR 分析评估 RAR)gamma、甾醇调节元件结合蛋白 (SREBP)-1 和 -2 以及肝脏 X 受体 (LXR)beta。激酶测定:GST-p62由大肠杆菌制备,0.5μg纯化的GST-p62蛋白用于体外AMPK磷酸化测定。使用 γS-ATP 通过非放射性同位素方法测定 AMPK 对 p62 蛋白的磷酸化。 AMPK 复合物是从 HEK293 细胞中免疫纯化的,用 Flag-AMPKβ1 和 HA-AMPKγ1 转染 myc-AMPKα1 野生型 (WT) 或 myc-AMPKα1 激酶死亡突变体 (KD、D157A)。将 AMPK 复合物添加到含有 20 mM HEPES、pH7.4、1 mM EGTA、0.4 mM EDTA、5 mM MgCl2、0.05 mM DTT、0.5 μg GST-p62、0.2 mM AMP 和 1 mM ATPγS 的反应混合物中。反应在37℃下进行30分钟,然后通过添加20mM EDTA终止。为了检测γS标记的p62蛋白,将反应产物用2.5 mM PNBM在室温下烷基化2小时,并使用抗硫代磷酸盐抗体通过蛋白质印迹分析产物。细胞测定:在与类胡萝卜素 (1 μM) 一起孵育的分化 Caco-2 细胞中,依折麦布 (10 mg/L) 抑制类胡萝卜素转运,对 ɑ-胡萝卜素和 β-胡萝卜素抑制 50%。此外,它还能抑制 β-隐黄质、番茄红素和叶黄素:玉米黄质 (1:1) 的转运。同时,依折麦布抑制胆固醇转运31%。依折麦布降低表面受体 SR-BI、ATP 结合盒转运蛋白、A 亚家族 (ABCA1)、Niemann-Pick C1 型样 1 蛋白 (NPC1L1) 和视黄酸受体 (RAR)γ、甾醇调节元件结合蛋白 SREBP 的表达-1 和 SREBP-2,以及肝 X 受体 (LXR)β。
|
| 体内研究 (In Vivo) |
在西方、低脂和无胆固醇饮食小鼠中,依泽替米贝分别将血浆胆固醇水平从 964 mg/dL 降低至 374 mg/dL、从 726 mg/dL 降低至 231 mg/dL 和从 516 mg/dL 降低至 178 mg/dL。依折麦布将西方饮食组小鼠的主动脉粥样硬化病变表面积从 20.2% 减少至 4.1%,将低脂胆固醇饮食组小鼠的主动脉粥样硬化病变表面积从 24.1% 减少至 7.0%。在西方和低脂胆固醇组中,依折麦布使颈动脉粥样硬化病变横截面积减少了 97%,在无胆固醇小鼠中减少了 91%。在 apoE-/- 小鼠中,依泽替米贝可抑制胆固醇吸收,降低血浆胆固醇,增加高密度脂蛋白水平,并抑制西方、低脂和无胆固醇饮食条件下动脉粥样硬化的进展。依折麦布可有效抑制胆固醇穿过肠壁的转运,从而降低高胆固醇血症临床前动物模型中的血浆胆固醇。在大鼠中建立了依泽替米贝消除肠道的外分泌胰腺功能,同时维持胆汁流动。依折麦布可降低胆固醇喂养仓鼠的血浆胆固醇和肝脏胆固醇积累,ED(50) 为 0.04 mg/kg。
|
| 酶活实验 |
使用大肠杆菌生产 GST-p62,并将 0.5 μg 纯化蛋白用于体外 AMPK 磷酸化测定。使用 S-ATP 的非放射性同位素方法用于测定 AMPK 对 p62 蛋白的磷酸化。从 HEK293 细胞中免疫纯化 AMPK 复合物,然后将 Flag-AMPKβ1 和 HA-AMPKγ1 转染至 myc-AMPKα1 野生型 (WT) 或 myc-AMPKα1 激酶死亡突变体 (KD、D157A) 细胞中。反应混合物含有 20 mM HEPES、pH 7.4、1 mM EGTA、0.4 mM EDTA、5 mM MgCl2、0.05 mM DTT、0.5 μg GST-p62、0.2 mM AMP 和 1 mM ATPS。然后将 AMPK 复合物添加到混合物中。反应在 37°C 下进行 30 分钟,然后添加 20 mM EDTA 结束反应。反应产物在室温下用 2.5 mM PNBM 烷基化 2 小时,以检测用 S 原子进行 γS 标记的 p62 蛋白 [1],然后使用抗硫代磷酸盐抗体进行蛋白质印迹分析。
|
| 细胞实验 |
Huh7 人肝细胞在 37°C、95% 空气/5% CO2 环境中使用含有 10% FBS、100 单位/mL 青霉素和 100 μg/mL 链霉素的高葡萄糖 DMEM 培养。依泽替米贝(10 μM,1 小时)和棕榈酸(0.5 mM,24 小时)在治疗或不治疗的情况下给予肝细胞[2]。
|
| 动物实验 |
Mice: We use male C57BL/6J mice that are ten weeks old. The three groups—normal chow diet, MCD diet with a vehicle treatment, or MCD diet with ezetimibe treatment—each containing 7–10 mice, are randomly chosen for the animals. The temperature was kept at 23±2°C, the humidity at 60%±10%, and there were 12-hour cycles of light and darkness for the mice. Ezetimibe 10 mg/kg is administered once daily by oral gavage to the MCD diet group for a period of four weeks. The same quantity of phosphate buffered saline was given orally to the chow and MCD diet with vehicle groups for a period of four weeks. Over the course of the therapy, weight is assessed once per week. The mice are sedated and killed after four weeks, and blood is extracted through a heart puncture. After being harvested, tissues are either fixed in formalin and then embedded in paraffin, or they are instantly frozen in liquid nitrogen and kept at -70°C.
Rats: The experiments are carried out in a particular pathogen-free facility with a 12 h light/dark cycle, using male OLETF (n=11) and age-matched LETO (n=3) rats. The OLETF rat is a model that depicts late-onset hyperglycemia and displays a chronic disease course, mild obesity, and clinical onset of diabetes mellitus. Animals have unrestricted access to food and water. Rats are randomized at 12 weeks of age and given either PBS or Ezetimibe (10 mg/kg per day) by stomach gavage for 20 weeks. The rats are fasted for the duration of the study, and then intraperitoneal Zoletil/Rompun is administered to put them to sleep. The liver is dissected, its tissues are immediately frozen in liquid nitrogen, and it is then stored at -80°C for later analysis after blood is drawn from the abdominal aorta. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Administration of a single 10-mg dose of ezetimibe in fasted adults resulted in peak plasma concentrations (Cmax) of 3.4-5.5 ng/mL within 4-12 hours (Tmax). The Cmax of the major pharmacologically-active metabolite, ezetimibe-glucuronide, was 45-71 ng/mL and its Tmax was 1-2 hours. Food consumption has minimal effect on ezetimibe absorption, but the Cmax is increased by 38% when administered alongside a high-fat meal. The true bioavailability of ezetimibe cannot be determined, as it is insoluble in aqueous media suitable for intravenous injection. Approximately 78% and 11% of orally administered radiolabelled ezetimibe are recovered in the feces and urine, respectively. Unchanged parent drug is the major component in feces and accounts for approximately 69% of an administered dose, while ezetimibe-glucuronide is the major component in urine and accounts for approximately 9% of an administered dose. High recovery of unchanged parent drug in feces suggests low absorption and/or hydrolysis of ezetimibe-glucuronide secreted in the bile. The relative volume of distribution of ezetimibe is 107.5L. There are no pharmacokinetic data available on the clearance of ezetimibe. Ezetimibe is the first member of a new class of selective cholesterol absorption inhibitors. The drug and its active glucuronide metabolite impair the intestinal reabsorption of both dietary and hepatically excreted biliary cholesterol through inhibition of a membrane transporter yet to be identified. Absorption of ezetimibe is rapid and not altered by food content following oral administration. The drug is not metabolized by the cytochrome P450 system but extensive glucuronidation takes place in the intestine. Consequently, plasma concentrations of ezetimibe represent approximately 10% of total ezetimibe in plasma. Enterohepatic recirculation observed for ezetimibe and its glucuronimide significantly increases the residence time of these compounds in the intestine, at their site of action. Elimination of ezetimibe glucuronimide appears impaired in elderly patients and patients with renal insufficiency with plasma concentrations increased 1.5- to 2-fold. So far, no drug interaction study has been associated with major changes in either the pharmokinetics of ezetimibe or coadministered drugs. Ezetimibe lowers plasma cholesterol levels by inhibiting the uptake of cholesterol in the intestine. Due to extensive enterohepatic circulation of ezetimibe, relative low doses are required to be effective. In blood and bile the majority of ezetimibe is present as a glucuronide-conjugate, which is formed in the enterocyte. Presently, it is not clear which mechanisms are responsible for this efficient enterohepatic circulation. Abcc2, Abcc3 and Abcg2 are ABC transporters, which are expressed in both liver and intestine and are capable of transporting glucuronidated compounds. The aim of this study was to investigate the contribution of these transporters in the enterohepatic cycling of ezetimibe-glucuronide (Ez-gluc). Transport studies were performed in plasma membrane vesicles from ABCC2, ABCC3 and ABCG2 expressing Sf21 insect cells. Furthermore, intestinal explants from wild-type and Abcc3-/- mice were used to study vectorial transport in an Ussing chamber setup. Finally, biliary excretion of Ez-gluc was measured in vivo after duodenal delivery of ezetimibe in wild-type, Abcc3-/-, Abcc2-/-, Abcg2-/- and Abcg2-/-/Abcc2-/- mice. ABCC3-, ABCC2- and ABCG2-mediated transport was dose dependently inhibited by Ez-gluc. In the Ussing chamber Ez-gluc recovered from the basolateral side was significantly reduced in duodenal (2.2%), in jejunal (23%) and in ileal (23%) tissue of Abcc3-/- compared to wild-type mice. Biliary excretion of Ez-gluc was significantly reduced in Abcc3-/- (34%), Abcc2-/- (56%) and Abcg2-/-/Abcc2-/- (2.5%) compared to wild-type mice. These data demonstrate that enterohepatic circulation of Ez-gluc strongly depends on the joint function of Abcc3, Abcc2 and Abcg2. It is not known whether ezetimibe is excreted into human breast milk. In rat studies, exposure to total ezetimibe in nursing pups was up to half of that observed in maternal plasma. After oral administration, ezetimibe is absorbed and extensively conjugated to a pharmacologically active phenolic glucuronide (ezetimibe-glucuronide). After a single 10-mg dose of Zetia to fasted adults, mean ezetimibe peak plasma concentrations (Cmax) of 3.4 to 5.5 ng/mL were attained within 4 to 12 hours (Tmax). Ezetimibe-glucuronide mean Cmax values of 45 to 71 ng/mL were achieved between 1 and 2 hours (Tmax). There was no substantial deviation from dose proportionality between 5 and 20 mg. The absolute bioavailability of ezetimibe cannot be determined, as the compound is virtually insoluble in aqueous media suitable for injection. Metabolism / Metabolites In humans, ezetimibe is rapidly and extensively metabolized via a phase II glucuronide conjugation reaction in the small intestine and liver to form its main phenolic metabolite, ezetimibe glucuronide. The main human liver and/or intestinal uridine 5′-diphosphate (UDP)-glucuronosyltransferase (UGT) enzymes responsible for the glucuronidation of ezetimibe were shown to be UGT1A1, 1A3, and 2B15 _in vitro_. Minimal phase I reaction involving oxidation of ezetimibe also occurs to form SCH 57871, and human jejunum microsomes also produced trace levels of a benzylic glucuronide (SCH 488128). Ezetimibe glucuronide accounts for 80-90% of the total circulating compound in plasma, and retains some pharmacological activity in inhibiting intestinal cholesterol uptake. In humans, ezetimibe and ezetimibe-glucuronide constitutes approximately 93% of the total drug in plasma. Plasma concentration-time profiles exhibit multiple peaks, suggestive of enterohepatic recycling, and about 20% of the drug distributed is reabsorbed due to enterohepatic recirculation. Ezetimibe is primarily metabolized in the small intestine and liver via glucuronide conjugation (a phase II reaction) with subsequent biliary and renal excretion. Minimal oxidative metabolism (a phase I reaction) has been observed in all species evaluated. In humans, ezetimibe is rapidly metabolized to ezetimibe-glucuronide. Ezetimibe and ezetimibe-glucuronide are the major drug-derived compounds detected in plasma, constituting approximately 10 to 20% and 80 to 90% of the total drug in plasma, respectively. Both ezetimibe and ezetimibe-glucuronide are eliminated from plasma with a half-life of approximately 22 hours for both ezetimibe and ezetimibe-glucuronide. Plasma concentration-time profiles exhibit multiple peaks, suggesting enterohepatic recycling. Following oral administration of (14)C-ezetimibe (20 mg) to human subjects, total ezetimibe (ezetimibe + ezetimibe-glucuronide) accounted for approximately 93% of the total radioactivity in plasma. After 48 hours, there were no detectable levels of radioactivity in the plasma. Approximately 78% and 11% of the administered radioactivity were recovered in the feces and urine, respectively, over a 10-day collection period. Ezetimibe was the major component in feces and accounted for 69% of the administered dose, while ezetimibe-glucuronide was the major component in urine and accounted for 9% of the administered dose. Ezetimibe has known human metabolites that include Ezetimibe-glucuronide. Biological Half-Life Both ezetimibe and ezetimibe-glucuronide display an approximate half-life of 22 hours. Both ezetimibe and ezetimibe-glucuronide are eliminated from plasma with a half-life of approximately 22 hours for both ezetimibe and ezetimibe-glucuronide. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Therapy with ezetimibe alone or in combination with other lipid lowering agents is associated with a low rate of serum enzyme elevations (0.5% to 1.5%), but most elevations are self-limited and not associated with jaundice or symptoms. In large randomized controlled trials, ezetimibe by itself has not been associated with a higher rate of serum ALT elevation than occurs with placebo therapy. However, the addition of ezetimibe to statin therapy has been associated with a slight increase in the likelihood of serum aminotransferase elevations or rates of discontinuation due to liver test abnormalities. Clinically apparent acute liver injury due to ezetimibe has been reported, but is rare. Furthermore, because this agent is often used in combination with other cholesterol lowering drugs, the role of ezetimibe in these reports is not always well defined. The latency to onset of clinically apparent liver injury attributed to ezetimibe has ranged from 2 to 10 months and the pattern of serum enzyme elevations has ranged from hepatocellular to cholestatic. Cases of autoimmune hepatitis-like injury have been described in patients taking the combination of ezetimibe and a statin, and the role of ezetimibe in these reactions is difficult to assign (Case 1). A single instance of vanishing bile duct syndrome due to ezetimibe has been described in a patient who continued on ezetimibe for several months despite presence of jaundice. Likelihood score: C (probable rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Data from 2 mothers indicate that levels of ezetimibe and its active metabolite appear in very low amounts in milk and serum levels in infants predicted by a pharmacokinetic model are considerably lower than in adults. Ezetimibe appears to be acceptable during breastfeeding. Ezetimibe in combination with a statin (e.g., atorvastatin, simvastatin) should be avoided in nursing mothers. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Ezetimibe and ezetimibe-glucuronide are >90% bound to human plasma proteins. The mean _in vitro_ protein binding ranged from 99.5% to 99.8% for ezetimibe and 87.8% to 92.0% for ezetimibe-glucuronide. Interactions Pharmacokinetic or pharmacodynamic interaction /with warfarin/ is unlikely, based on one small study. Increased international normalized ratio (INR) with concomitant use of ezetimibe and warfarin has been reported during postmarketing experience; however, most patients also were receiving other drugs. Monitor INR if ezetimibe is initiated in a patient receiving warfarin. Potential pharmacokinetic interaction (increased peak plasma ezetimibe concentration and AUC, increased cyclosporine AUC). The degree of exposure to ezetimibe may be greater in patients with severe renal insufficiency. Risk of myopathy/rhabdomyolysis is increased following concomitant administration of the fixed combination of ezetimibe and simvastatin (particularly at higher dosages) with cyclosporine. Because of increased exposure to ezetimibe and cyclosporine, use concomitantly with caution and monitor cyclosporine concentrations. If used concomitantly, dosage of the fixed-combination preparation should not exceed 10 mg of ezetimibe and 10 mg of simvastatin daily. Potential pharmacokinetic (decreased AUC of ezetimibe) and pharmacodynamic (reduced LDL-cholesterol lowering effect) interaction. Ezetimibe should be administered at least 2 hours before or at least 4 hours after administration of the bile acid sequestrant. Pharmacokinetic interaction (increased plasma ezetimibe concentrations) observed when used concomitantly with fenofibrate or gemfibrozil. Fibric acid derivatives may increase cholesterol excretion into bile, leading to cholelithiasis, and ezetimibe has been shown to increase cholesterol in the gall bladder bile in animals. In clinical studies, cholecystectomy has been reported in 1.7% of patients receiving ezetimibe concomitantly with fenofibrate and in 0.6% of those receiving fenofibrate monotherapy. Concomitant use with a fibric acid derivative other than fenofibrate currently is not recommended pending further accumulation of data in humans. If cholelithiasis is suspected in a patient receiving ezetimibe with fenofibrate, gallbladder studies should be performed, and alternative antilipemic therapy should be considered. For more Interactions (Complete) data for Ezetimibe (6 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Ezetimibe is used alone or in combination with other antilipemic agents (i.e., a hydroxymethylglutaryl-coenzyme A [HMG-CoA] reductase inhibitor (statin), fenofibrate) as an adjunct to dietary therapy in the treatment of primary hypercholesterolemia and mixed dyslipidemia, homozygous familial hypercholesterolemia, and/or homozygous familial sitosterolemia. /Included in US product label/ Ezetimibe is used alone or in combination with a statin as an adjunct to dietary therapy to decrease elevated serum total cholesterol, low-density lipoprotein (LDL)-cholesterol, and apolipoprotein B (apo B) concentrations in the treatment of primary (heterozygous familial and nonfamilial) hypercholesterolemia. Ezetimibe in fixed combination with simvastatin is used as an adjunct to dietary therapy to decrease elevated serum total cholesterol, LDL-cholesterol, apo B, triglyceride, and non-HDL-cholesterol concentrations, and to increase HDL-cholesterol concentrations in the treatment of primary hypercholesterolemia or mixed dyslipidemia. Ezetimibe also is used in combination with fenofibrate as an adjunct to dietary therapy to decrease elevated serum total cholesterol, LDL-cholesterol, apo B, and non-HDL-cholesterol concentrations in the treatment of mixed dyslipidemia. /Included in US product label/ Ezetimibe is used as an adjunct to dietary therapy to decrease elevated serum sitosterol and campesterol concentrations in patients with homozygous familial sitosterolemia. /Included in US product label/ Ezetimibe may be used in combination with atorvastatin or simvastatin to decrease elevated serum total and LDL-cholesterol concentrations in patients with homozygous familial hypercholesterolemia as an adjunct to other lipid-lowering therapies (e.g., plasma LDL apheresis) or when such therapies are not available. /Included in US product label/ This is a retrospective review of all pediatric patients who received ezetimibe monotherapy as treatment for hypercholesterolemia and for whom follow-up clinical and lipid results were available. Of 36 identified patients, 26 had lipoprotein profiles suggestive of familial hypercholesterolemia (FH), and 10 had profiles suggestive of familial combined hyperlipidemia (FCHL). After a mean 105 days of treatment with ezetimibe (range, 32-175 days), total cholesterol (TC) levels decreased from 7.3 +/- 1.0 mmol/L to 5.7 +/- 1.0 mmol/L (P < .0001), and low-density lipoprotein cholesterol (LDL-C) levels decreased from 5.3 +/- 0.9 mmol/L to 3.9 +/- 0.8 (P < .0001) in patients with FH. In patients with FCHL, TC levels decreased from 6.4 +/- 2.0 mmol/L to 5.6 +/- 0.4 mmol/L (P < or = .002), and LDL-C levels decreased from 4.7 +/- 1.0 mmol/L to 3.8 +/- 0.6 mmol/L (P < or = .005). For all patients, the mean decrease in individual LDL-C values was 1.5 +/- 0.9 mmol/L or 28%. There was no significant change in triglyceride or high-density lipoprotein cholesterol levels with ezetimibe. Patients were maintained on ezetimibe with no adverse effects attributable to the medication for as long as 3.5 years. At a mean of 13.6 months (range, 1-44 months) after the initiation of ezetimibe, LDL-C levels remained decreased at 4.0 +/- 0.6 mmol/L. In this small retrospective series of children and adolescents with hypercholesterolemia, ezetimibe was safe and effective in lowering LDL-C levels. Drug Warnings Ezetimibe, in combination with a hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor (statin), is contraindicated in patients with active liver disease or unexplained, persistent increases in serum aminotransferase (transaminase) concentrations. In the Zetia controlled clinical trials database (placebo-controlled) of 2396 patients with a median treatment duration of 12 weeks (range 0 to 39 weeks), 3.3% of patients on Zetia and 2.9% of patients on placebo discontinued due to adverse reactions. The most common adverse reactions in the group of patients treated with Zetia that led to treatment discontinuation and occurred at a rate greater than placebo were: Arthralgia (0.3%); dizziness (0.2%); and gamma-glutamyltransferase increased (0.2%) The most commonly reported adverse reactions (incidence =2% and greater than placebo) in the Zetia monotherapy controlled clinical trial database of 2396 patients were: upper respiratory tract infection (4.3%), diarrhea (4.1%), arthralgia (3.0%), sinusitis (2.8%), and pain in extremity (2.7%). In the Zetia + statin controlled clinical trials database of 11,308 patients with a median treatment duration of 8 weeks (range 0 to 112 weeks), 4.0% of patients on Zetia + statin and 3.3% of patients on statin alone discontinued due to adverse reactions. The most common adverse reactions in the group of patients treated with Zetia + statin that led to treatment discontinuation and occurred at a rate greater than statin alone were: Alanine aminotransferase increased (0.6%) Myalgia (0.5%) Fatigue, aspartate aminotransferase increased, headache, and pain in extremity (each at 0.2%) The most commonly reported adverse reactions (incidence =2% and greater than statin alone) in the Zetia + statin controlled clinical trial database of 11,308 patients were: nasopharyngitis (3.7%), myalgia (3.2%), upper respiratory tract infection (2.9%), arthralgia (2.6%) and diarrhea (2.5%). In post-marketing experience with Zetia, cases of myopathy and rhabdomyolysis have been reported. Most patients who developed rhabdomyolysis were taking a statin prior to initiating Zetia. However, rhabdomyolysis has been reported with Zetia monotherapy and with the addition of Zetia to agents known to be associated with increased risk of rhabdomyolysis, such as fibrates. Zetia and any statin or fibrate that the patient is taking concomitantly should be immediately discontinued if myopathy is diagnosed or suspected. The presence of muscle symptoms and a CPK level >10 times the upper limit of normal (ULN) indicates myopathy. For more Drug Warnings (Complete) data for Ezetimibe (15 total), please visit the HSDB record page. Pharmacodynamics Ezetimibe was shown to reduce the levels of total cholesterol (total-C), low-density lipoprotein cholesterol (LDL-C), apoprotein B (Apo B), non-high-density lipoprotein cholesterol (non-HDL-C), and triglycerides (TG), and increase high-density lipoprotein cholesterol (HDL-C) in patients with hyperlipidemia. This therapeutic effect was more profound when ezetimibe was co-administered with a statin or fenofibrate compared to either treatment alone. In clinical trials involving patients with homozygous and heterozygous familial hypercholesterolemia and in those with sitosterolemia, a recommended therapeutic dose of ezetimibe was effective in reducing the LDL levels by 15-20% while increasing HDL-C by 2.5-5%. The effects of increased exposure to ezetimibe secondary to moderate-severe hepatic impairment have not been assessed - patients meeting these criteria should avoid the use of ezetimibe. Post-marketing reports indicate the potential for myopathy and rhabdomyolysis in patients taking ezetimibe, and this risk appears to be exacerbated in patients concurrently receiving, or having recently received, statin therapy. |
| 分子式 |
C24H21F2NO3
|
|---|---|
| 分子量 |
409.4
|
| 精确质量 |
409.148
|
| 元素分析 |
C, 70.41; H, 5.17; F, 9.28; N, 3.42; O, 11.72
|
| CAS号 |
163222-33-1
|
| 相关CAS号 |
Ezetimibe;163222-33-1
|
| PubChem CID |
150311
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
654.9±55.0 °C at 760 mmHg
|
| 熔点 |
164-166℃
|
| 闪点 |
349.9±31.5 °C
|
| 蒸汽压 |
0.0±2.1 mmHg at 25°C
|
| 折射率 |
1.624
|
| LogP |
3.26
|
| tPSA |
60.77
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
567
|
| 定义原子立体中心数目 |
3
|
| SMILES |
FC1C([H])=C([H])C(=C([H])C=1[H])N1C([C@]([H])(C([H])([H])C([H])([H])[C@@]([H])(C2C([H])=C([H])C(=C([H])C=2[H])F)O[H])[C@@]1([H])C1C([H])=C([H])C(=C([H])C=1[H])O[H])=O
|
| InChi Key |
OLNTVTPDXPETLC-XPWALMASSA-N
|
| InChi Code |
InChI=1S/C24H21F2NO3/c25-17-5-1-15(2-6-17)22(29)14-13-21-23(16-3-11-20(28)12-4-16)27(24(21)30)19-9-7-18(26)8-10-19/h1-12,21-23,28-29H,13-14H2/t21-,22+,23-/m1/s1
|
| 化学名 |
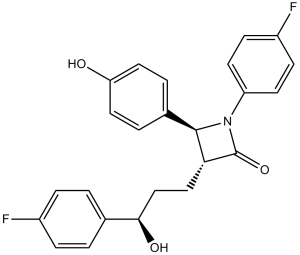
(3R,4S)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one
|
| 别名 |
SCH-58235; SCH 58235; SCH-58235; SCH58235; trade names: Zetia, Ezetrol
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|---|
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4426 mL | 12.2130 mL | 24.4260 mL | |
| 5 mM | 0.4885 mL | 2.4426 mL | 4.8852 mL | |
| 10 mM | 0.2443 mL | 1.2213 mL | 2.4426 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
CHOlesterol Lowering and Residual Risk in Type 2 Diabetes
CTID: NCT04369664
Phase: Phase 4 Status: Completed
Date: 2024-10-17
|
|
|
|