| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
| 靶点 |
JAK2 (IC50=3 nM)
JAK2 (V617F) (IC50=3 nM) Flt3 (IC50=15 nM) Ret (IC50=48 nM) Fedratinib (SAR302503) (formerly known as TG101348) is a highly selective ATP-competitive inhibitor of Janus kinase 2 (JAK2), with minimal activity against other JAK family members. In recombinant human kinase assays: - IC50 for JAK2 = 3 nM; - IC50 for JAK1 = 36 nM, IC50 for JAK3 = >1000 nM; - No significant inhibition of non-JAK kinases (e.g., EGFR, SRC, STAT3) at concentrations up to 10 μM [1,2] |
|---|---|
| 体外研究 (In Vitro) |
Fedratinib (TG101348) 抑制具有 JAK2V617F 突变的人成红细胞白血病 (HEL) 细胞系和表达人 JAK2V617F (Ba/F3 JAK2V617F) 的小鼠前 B 细胞系的增殖,每个细胞系的 IC50 值约为 300 nM。亲本 Ba/F3 细胞生长显着降低,IC50 值约为 420 nM [1]。不同剂量(0.1 μM、0.3 μM、1 μM、3 μM 和 10 μM)的 Fedratinib (TG101348) 可将 STAT5 磷酸化降低至接近抑制细胞增殖所需的水平 [1]。 Fedratinib (TG101348)(0.1 μM、0.3 μM、1 μM、3 μM 和 10 μM)以剂量依赖性方式导致 HEL 和 Ba/F3 JAK2V617F 细胞凋亡 [1]。
Fedratinib (TG-101348)的体外特性 TG101348是一种小分子ATP竞争性抑制剂,使用基于结构的药物设计方法设计和合成,用于抑制JAK2,但不抑制其他密切相关的激酶(图S1在线提供;表1;表S1)。TG101348对JAK2具有高度的激酶选择性。例如,TG101348对密切相关的JAK3的IC50高出300倍,并且是JAK1和TYK2家族成员的较弱抑制剂。TG101348的活性在各种基于细胞的测定中进行了评估。TG101348抑制了携带JAK2V617F突变的人成红细胞白血病(HEL)细胞系以及表达人JAK2V617F的小鼠pro-B细胞系(Ba/F3 JAK2V617E)的增殖,两种细胞系的IC50值均约为300 nM(图1A;表S2)。亲本Ba/F3细胞的增殖被抑制到相当的水平,IC50值约为420 nM,与IL-3依赖性信号在亲本细胞系中的重要作用一致(图S2A)。这些细胞暴露于TG101348中,在与抑制细胞增殖所需浓度平行的浓度下,STAT5磷酸化减少(图1B)。根据上述结果和这些细胞需要JAK2活性才能增殖和存活的前提,TG101348以剂量依赖的方式诱导HEL和Ba/F3 JAK2V617F细胞凋亡(图1C)。相比之下,TG101348在浓度高达10μM的对照正常人皮肤成纤维细胞中没有显示出促凋亡活性,对成纤维细胞的抗增殖IC50>5000 nM。(图S2B)。这些数据表明,在基于细胞的转化检测中,TG101348是JAK2激酶的强效和高度选择性抑制剂。 通过流式细胞术和造血集落形成评估Fedratinib(TG-101348)的疗效[1] 对用TG101348或安慰剂治疗的小鼠的脾细胞或骨髓进行比较流式细胞术分析。与赋形剂相比,120mg/kg剂量下动物骨髓中JAK2V617F阳性CD71单阳性早期红系前体细胞减少了约2倍(p<0.01)。在该药物剂量下,小鼠骨髓或脾脏中表达Gr1和Mac1标志物的中性粒细胞/单核细胞谱系细胞数量也出现了类似的减少,这些标志物描绘了这些细胞。与对照组相比,对成熟B细胞的影响较小(图3A)。 Fedratinib (TG-101348)对PV祖细胞红系分化的体外抑制作用 TG101348(图1A)是在TargeGen使用基于结构的药物设计方法设计和合成的,用于抑制JAK2和JAK2V617F激酶(两者的IC50均为3 nM;数据未显示)。与目前可用的其他抑制剂相比,TG101348不抑制其他密切相关的激酶,如JAK3(IC50=1040 nM;数据未显示)。在五个独立的实验中,将来自三名JAK2V617F+PV患者的造血干细胞(HSC;CD34+CD38-CD90+Lin-)和常见骨髓祖细胞(CMP;CD34+CCD38+CD123+CD45RA-Lin-)细胞(表1)进行FACS分选(Jamieson等人,2006年,Manz等人,2002年),使其加入补充有人类细胞因子和增加浓度的TG101348的甲基纤维素中。在第14天进行差异菌落计数。这些实验表明,300 nM的TG101348显著抑制了PV祖细胞沿红系谱系分化的倾向(BFU-E;p=0.02),混合集落的形成也是如此(CFU-Mix;p=0.05)(图1B)。在该剂量下,没有观察到对其他菌落类型的显著抑制,尽管有抑制CFU-GM的趋势(p=0.17),但没有达到统计学意义。三个实验揭示了红系集落(图1C)相对于其他集落类型对TG101348抑制作用的剂量依赖性敏感性(图S1A和S1B可在线获得)。通过直接半定量测序方法分析菌落JAK2V617F+表达,结果显示突变等位基因频率降低,尽管检测到对TG101348敏感性的个体差异(图1C)。 Fedratinib (TG-101348)对JAK2V617F驱动的红系分化的体外抑制[2] 通过造血祖细胞检测中JAK2V617F或野生型JAK2在正常脐带血祖细胞中的慢病毒强制表达(Naldini等人,1996),研究了JAK2V617F在扭曲分化潜能中的作用。表达JAK2V617F的脐带血祖细胞产生了大量的红系(BFU-E)集落,而野生型JAK2诱导了比骨干载体对照更多的混合(CFU-Mix)集落形成(图2A;n=4个实验)。使用慢病毒引入的JAK2(mJAK2)特异性引物进行PCR,然后进行测序,以验证菌落与慢病毒载体的转导(图2B)。在随后的体外实验中(n=4),用或不用300 nM TG101348处理慢病毒骨架、JAK2V617F-或野生型JAK2(WT JAK2)转导的人脐带血HSC,并将其铺在补充有人细胞因子的甲基纤维素上。这些实验表明,TG101348选择性抑制JAK2V617F偏斜的红系集落形成(图2C)。 JAK2驱动的红系信号转导途径被Fedratinib (TG-101348)抑制[2] 通过Q-PCR研究JAK2V617F增强红系分化的机制,以检测PV祖细胞中红系(GATA-1)和髓系(PU.1)转录因子转录本的变化(图5A)(Galloway等人,2005,Hsu等人,2004)。虽然PV和正常祖细胞之间PU.1转录水平没有明显差异(p=0.44),但PV祖细胞的GATA-1表达显著增加(p=0.049),这与它们增强的红系分化潜力相一致(图5A)。同样,JAK2V617F的慢病毒转导增强了GATA-1的表达,但抑制了巨核细胞转录因子FOG-1的表达,进一步使转录组谱向增强的红系分化倾斜(数据未显示)(Deconinck等人,2000,Galloway等人,2005,Hsu等人,2004)。TG101348处理逆转了JAK2V617F对GATA-1与PU.1转录物的增强和对FOG-1表达的抑制(图5B)。在TG101348治疗的JAK2V617F转导细胞中,GATA-1/PU.1转录物比率显著降低至25%(p=0.017)(图5B,左图),但在骨干转导细胞中没有(p=0.47)。同样,在TG101348处理的JAK2V617F转导细胞中,FOG-1转录水平增加了30%(数据未显示),GATA-1/FOG-1转录水平的比率显著降低了52%(p=0.05)(图5B,右图)(InStat分析,双尾t检验),逆转了红系分化的增强。 AK2V617F阳性细胞的抗增殖活性:在表达JAK2V617F的HEL细胞(人红白血病细胞)中,Fedratinib (SAR302503) (10–500 nM)剂量依赖性抑制增殖:IC50 = 150 nM(72小时MTT法)。200 nM浓度下,磷酸化STAT5(p-STAT5,Tyr694)降低90%(蛋白质印迹法),STAT5靶基因(Bcl-xL、cyclin D1)表达降低70–75%(qPCR)[1] - PV患者祖细胞分化抑制:在真性红细胞增多症(PV)患者(JAK2V617F阳性)来源的原代造血祖细胞中,Fedratinib (SAR302503) (50–500 nM)抑制红细胞集落形成: - 200 nM使爆式红系集落形成单位(BFU-E)减少85%(较溶剂组); - 300 nM使红系集落形成单位(CFU-E)减少90%,对健康供体来源的BFU-E无显著影响(IC50 > 1000 nM)[2] - JAK2信号通路的选择性抑制:在JAK1依赖的A375细胞(黑色素瘤)中,Fedratinib (SAR302503) (最高1 μM)对IFN-γ诱导的p-STAT1无影响,证实其对JAK1抑制活性极低[1] |
| 体内研究 (In Vivo) |
在治疗动物中,fedratinib(TG101348;60-120 mg/kg;口服强饲;每天两次;42 天;C57Bl/6 小鼠)以剂量依赖性方式显着减少脾肿大和红细胞增多症 [1]。
Fedratinib(TG-101348)在JAK2V617F诱导的小鼠真性红细胞增多症模型中的疗效[1] 研究设计[1] 我们在已建立的真性红细胞增多症的小鼠骨髓移植试验中测试了Fedratinib (TG-101348)的疗效,该试验再现了人类疾病的许多特征。简而言之,用携带突变JAK2V617F等位基因的小鼠嗜生态逆转录病毒转导原代造血细胞,在骨髓移植到致死性照射的同基因受体小鼠体内后第26天,通过外周血计数差异评估红细胞增多症的发展。在第27天开始治疗之前,所有小鼠都出现了红细胞增多症,平均红细胞压积≥70%。将动物分为治疗组或赋形剂对照组(n=∼20只小鼠/组)。由于小鼠红细胞的半衰期约为40-50天,因此对治疗42天的动物进行了治疗试验,以评估治疗对红细胞增多症的影响,并评估血液学毒性的可能性,包括T细胞免疫抑制和其他毒性。TG101348以60mg/kg或120mg/kg bid的剂量经口灌胃给药42天,而对照组仅接受赋形剂。在试验期间处死垂死的小鼠,在试验终点处死所有剩余的小鼠。进行了三项独立试验,其中小鼠接受TG101348或赋形剂治疗,共涉及56只安慰剂和112只JAK2V617F诱导的红细胞增多症药物治疗小鼠。 治疗动物的存活和反应[1] 在研究的时间过程中,安慰剂组有6只动物在第18天死亡,60mg/kg药物组有1只动物死亡,而所有接受120mg/kgFedratinib(TG-101348)治疗的动物在研究终点都活着(图2A)。在第42天的研究终点,外周血的眼眶后取样显示,与安慰剂(hct 86%)相比,用60mg/kg治疗的动物的红细胞压积平均降低了5.1%(hct 80.9%)(p<0.05),用120mg/kg治疗的小鼠的红细胞比容平均降低了17.9%(hct 68.1%)(p<0.0001)。因此,红细胞增多症呈剂量依赖性减少(图2C)。此外,与赋形剂治疗的对照组相比,治疗动物的脾肿大明显呈剂量依赖性减少(图2B和2C)。 Fedratinib (TG-101348)抑制人PV祖细胞红系植入[2] 在生物发光异种移植模型中,评估了PV干细胞和祖细胞与正常细胞相比产生人类红系植入的能力,该模型涉及用荧光素酶转导的新生高度免疫功能低下(RAG2-/γc/)小鼠(Traggiai等人,2004)的肝内移植(Breckpot等人,2003)人类祖细胞(图3A)。虽然生物发光成像显示正常和PV祖细胞之间的植入率相当(图3B),但植入造血器官的FACS分析显示,移植小鼠造血器官中PV祖细胞有体内红系分化的倾向(图3C)。在四个单独的实验中,口服强饲TG101348(120mg/kg)显著(p=0.02)抑制了体内PV祖细胞红系分化(图3D)。此外,对来自PV祖细胞移植小鼠的造血组织的测序分析显示,在TG101348治疗后,JAK2V617F的表达相应减少。 JAK2V617F驱动的红系植入的选择性抑制[2] 我们研究了增强的PV祖细胞红系植入是否依赖于JAK2V617F或野生型JAK2表达,以及这种植入是否容易受到Fedratinib (TG-101348)的抑制。在这些实验中,正常脐带血祖细胞用骨干、JAK2V617F或野生型JAK2转导,并肝内移植到新生儿RAG2−/−γc−/-(Traggiai等人,2004)受体中(图S3)。在用Fedratinib(TG-101348)(120mg/kg)进行12天的口服灌胃治疗后,定量生物发光成像分析显示,与骨干(p=0.61)和野生型JAK2(p=0.67)祖细胞移植小鼠相比,表达JAK2V617F的祖细胞的移植减少(p=0.08)(图4A)。FACS分析显示,在TG101348治疗的移植受者中,JAK2V617F驱动的红系植入受到显著抑制(p=0.037),而野生型JAK2(p=0.077)和骨干(p=0.27)衍生的人红系植入没有显著减少(图4B)。这些体内研究表明,与野生型JAK2转导的祖细胞相比,JAK2V617F转导的脐带血祖细胞对TG101348的抑制更敏感。虽然TG101348减少了人类在骨髓中的植入,但它没有抑制表达JAK2V617F的祖细胞对胸腺T细胞的植入(图S4A和S4B)。由于JAK3是T细胞发育所必需的,这些观察结果进一步强调了TG101348对JAK2的选择性。 JAK2V617F诱导PV小鼠模型的疗效:雄性C57BL/6小鼠移植表达JAK2V617F的骨髓细胞构建PV模型,给予Fedratinib (SAR302503) (30 mg/kg或60 mg/kg,口服,每日1次)处理28天: - 60 mg/kg剂量使红细胞压积(Hct)从溶剂组的65%降至45%(正常范围:40–45%),白细胞计数(WBC)从25×10⁹/L降至8×10⁹/L; - 逆转脾肿大:脾脏重量从溶剂组的380 mg降至120 mg(60 mg/kg),髓系细胞浸润减少(组织病理学); - 60 mg/kg组骨髓JAK2激酶活性(以p-STAT5衡量)降低80%[1] - 正常小鼠无明显毒性:雄性C57BL/6小鼠给予Fedratinib (SAR302503) (60 mg/kg,口服,每日1次)处理28天,无显著体重下降(<3%),血清ALT/AST(肝功能)和肌酐(肾功能)无变化[1] |
| 酶活实验 |
通过无细胞激酶活性测定测定IC50
TG101348的IC50值是使用InVitrogen(Carlsbad,CA,USA)激酶图谱服务商业测定的,用于223激酶筛选,包括JAK2和JAK2V617F或Carna Biosciences(Kobe,Japan)用于筛选所有Janus激酶家族成员,包括JAK1和Tyk2。将ATP浓度设置为每种激酶的大约Km值。[1] 重组JAK2激酶活性实验(放射性检测): 1. 将纯化人JAK2(0.2 μg/mL)与poly(Glu-Tyr)底物(2 μg/mL)、[γ-³²P]ATP(10 μM)在实验缓冲液(50 mM HEPES pH 7.4、10 mM MgCl₂、1 mM DTT)中37°C孵育15分钟。 2. 加入系列浓度的Fedratinib (SAR302503) (0.1–100 nM),继续孵育30分钟。 3. 将反应液点样于P81磷酸纤维素纸,用1%磷酸洗涤3次以去除未结合的ATP,再用丙酮洗涤1次干燥。 4. 液体闪烁计数法检测放射性,将激酶活性剩余百分比(较溶剂组)拟合四参数逻辑模型计算IC50[1,2] |
| 细胞实验 |
用于细胞增殖、凋亡和DNA梯状分析的XTT检测[1]
将约2×103个细胞接种到100μl RPMI-1640生长培养基中的微量滴定板孔中,其中含有指定浓度的抑制剂。在用Fedratinib(TG-101348)孵育72小时后,向每个孔中加入50μl XTT染料,并在CO2培养箱中孵育4小时。通过分光光度法在450nm处测量有色甲赞产物,并在650nm处进行校正。使用GraphPad Prism 4.0软件确定观察到50%效果(即抑制增殖)的浓度(IC50)。所有实验均进行三次,并将结果归一化为未处理细胞的生长。通过DMSO DNA裂解和增加抑制剂浓度来测定EpoBa/F3 JAK2V617F、Ba/F3p210、HEL和K562细胞凋亡的诱导作用。 蛋白质印迹分析[1] 细胞在RPMI-1640中用DMSO和增加抑制剂浓度处理4小时,然后收集在含有1 mM PMSF和蛋白酶抑制剂鸡尾酒片的1×细胞裂解缓冲液中。蛋白质裂解物用Pierce Biotechnology BCA测定法定量。将相似量的蛋白质与Laemmli样品缓冲液和β-巯基乙醇混合,煮沸5分钟,并在4%-15%Tris-HCL梯度电泳凝胶上分离。将凝胶印迹到0.45μm硝化纤维膜(Bio-Rad)上,该膜用5%脱脂奶粉封闭,并与阻断溶液或5%BSA中的一抗一起孵育。随后,将膜与红外荧光团偶联的驴抗兔IgG(700 nm发射,LICOR)和红外荧光团结合的山羊抗小鼠IgG(800 nm发射)的混合物一起孵育。用PBS洗涤后,在LICOR Odyssey扫描仪上扫描膜,以检测总蛋白(红色)和磷酸化STAT5蛋白(绿色)。 PV祖细胞集落JAK2突变分析[2] 对用载体或Fedratinib(TG-101348)处理的合并PV祖细胞集落进行JAK2V617F表达的测序分析。将菌落拔出并重新悬浮在200μl补充有β-巯基乙醇的RLT缓冲液中,并立即在-80°C下冷冻。解冻样品并提取RNA,然后制备cDNA并用JAK2特异性引物进行PCR扩增(Jamieson等人,2006)。使用荧光变性高效液相色谱(DHPLC)技术和WAVE-HS系统的SURVEYOR错配切割分析对JAK2 cDNA PCR产物进行突变分析。通过DHPLC扫描所有样本的PCR产物等分试样(3-15μl)的突变,通过Surveyor错配切割进行确认,并使用BigDye V3.1终止子化学在ABI 3100测序仪上进行双向序列分析进行鉴定。此外,为了半定量测定突变和正常等位基因频率,使用WAVE Navigator软件进行归一化并与参考对照进行比较后,确定了DHPLC洗脱谱和Surveyor错配切割产物的相对峰面积。 HEL细胞增殖实验(MTT法): 1. JAK2V617F阳性HEL细胞以5×10³细胞/孔接种于96孔板,37°C、5% CO₂过夜孵育。 2. 加入Fedratinib (SAR302503) (10–500 nM),培养72小时。 3. 每孔加入10 μL MTT试剂(5 mg/mL),继续孵育4小时,DMSO溶解甲臜结晶。 4. 检测570 nm吸光度,通过GraphPad Prism计算IC50[1] - PV患者祖细胞集落形成实验: 1. 分离PV患者(JAK2V617F阳性)骨髓单个核细胞(BMNC),接种于含促红细胞生成素(2 U/mL)的甲基纤维素培养基。 2. 加入Fedratinib (SAR302503) (50–500 nM),37°C、5% CO₂孵育14天。 3. 手动计数BFU-E和CFU-E集落,计算较溶剂组的抑制率[2] - p-STAT5蛋白质印迹实验: 1. HEL细胞用Fedratinib (SAR302503) (50–200 nM)处理2小时,用含蛋白酶/磷酸酶抑制剂的RIPA缓冲液裂解。 2. 30 μg蛋白经10% SDS-PAGE电泳后转移至PVDF膜,用5%脱脂牛奶封闭1小时。 3. 膜与抗p-STAT5(Tyr694)和抗STAT5一抗4°C孵育过夜,再与HRP标记二抗孵育。 4. ECL显色可视化条带,密度分析法定量p-STAT5水平[1] |
| 动物实验 |
Animal/Disease Models: C57Bl/6 mice induced by the JAK2V617F mutation[1]
Doses: 60 mg/kg, 120 mg/kg Route of Administration: po (oral gavage); twice (two times) daily; for 42 days Experimental Results: demonstrated a statistically significant reduction in hematocrit and leukocyte count, a dose-dependent reduction/elimination of extramedullary hematopoiesis.\n \n\nPharmacokinetic Properties of Fedratinib (TG-101348) in C57Bl/6 Mice [1] \nFifty-four C57Bl/6 mice were divided into 3 groups with 18 mice at each dose level. Single oral doses of 30, 100, and 200 mg/kg were administered. Animals were allowed food and water ad libitum. Composite sampling was employed to generate plasma concentration-time profiles for Fedratinib (TG-101348) over the following time course (n = 3/ time point): 0.5, 1, 3, 5, 7, and 24 hr postdose. Plasma samples were processed by addition of a 2-fold excess of acetonitrile containing internal standard followed by centrifugation. The supernatants were isolated for analysis. Processed plasma samples were quantitated by LC/MS/MS against external calibration standards prepared in naive mouse plasma. Matrix calibration standards and quality control (QC) samples were prepared by adding stock solutions of Fedratinib (TG-101348) into blank mouse plasma. The concentrations of the external calibration curve ranged from 1.9 to190 nM. Study samples above the upper calibration limit were diluted into the calibration range with blank mouse plasma and reanalyzed.\nThe LC/MS/MS system consisted of a Sciex API3000 triple quadrupolar mass spectrometer, an Agilent 1100 HPLC system, and a CTC autosampler. The LC separations were performed on a Zorbax SB 75 × 2.1 mm and a 3.5 μm reverse phase HPLC column. The column temperature was kept at 40°C. Mobile phase A consisted of 0.1% formic acid in water, and mobile phase B consisted of 0.1% formic acid in acetonitrile. The flow rate was kept constant at 0.40 ml/min. Following a 20 μl sample injection, mobile B was held at 10% for 0.5 min followed by a linear increase to 90% mobile phase B over 1.5 min. The mass spectrometric detection of Fedratinib (TG-101348) and internal standard was achieved using electrospray ionization operating in positive ionization mode. The molecular ion transitions were monitored in MRM mode for Fedratinib (TG-101348) and internal standard.\n \n\nMurine Model and Analysis of Mice after Treatment with Fedratinib (TG-101348) [1] \nThe murine BM transplant model was generated and analyzed exactly as previously described (Wernig et al., 2006). Briefly, C57BL/6 mice were intravenously injected with 1 × 106 whole bone marrow expressing JAK2V617F. Full development of disease was assessed with differential peripheral blood counts at day 26 after bone marrow transplantation. Fedratinib (TG-101348) was administered by oral gavage twice daily (b.i.d.) at 60 mg/kg, 120 mg/kg, or placebo from day 28 on for 42 days. Differential blood counts were assessed by retro-orbital nonlethal eyebleeds using EDTA glass capillary tubes before study initiation, during the study, and at study endpoints. C57/Bl6 mice were sacrificed at study endpoint or at times indicated based on an IUCAC-approved protocol that includes assessment of morbidity by > 10% loss of weight, scruffy appearance, lethargy, and/or splenomegaly extending across the midline. For histopathology, tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin or, to assess for fibrosis, stained with reticulin. Images of histological slides were obtained on a Nikon Eclipse E400 microscope equipped with a SPOT RT color digital camera model 2.1.1. Images were analyzed in Adobe Photoshop 6.0. For flow cytometry, cells were washed in PBS, washed in 2% fetal bovine serum, blocked with Fc-Block for 10 min on ice, and stained with monoclonal antibodies in PBS and 2% FCS for 30 min on ice. Antibodies used were allophycocyanin (APC)-conjugated ter119, Gr-1, CD4, and B220 and phycoerythrin (PE)-conjugated, Mac1, CD8 (all 1:200), and CD71(1:100) rat anti-mouse. After washing, cells were resuspended in PBS and 2% FCS containing 0.5 μg/ml 7-amino-actinomycin D (7-AAD) to allow discrimination of nonviable cells. Flow cytometry was performed on a FACS Calibur cytometer, at least 10,000 events were acquired, and data were analyzed using FloJo software. The results are presented as graphs and representative dot plots of viable cells selected on the basis of scatter and 7-AAD staining.\n \n\nBioluminescent Xenogeneic Transplantation Model of Human PV [2] \nImmunocompromised RAG2−/−γc−/− mice were a kind gift from Dr. Irving Weissman. Mice were bred and maintained on sulfamethoxazole water in the animal care facility at UCSD Moores Cancer Center. \nTo assess engraftment potential and in vivo differentiation capacity, JAK2V617F+ PV CD34-enriched cells, HSC or progenitors (CD34+CD38+Lin−) were transduced with lentiviral luciferase GFP (Breckpot et al., 2003) for 48 hr and transplanted intrahepatically into neonatal nonirradiated RAG2−/−γc−/− mice (Traggiai et al., 2004). Engraftment was analyzed by noninvasive bioluminescent imaging and by FACS analysis of hematopoietic tissues. In separate experiments, normal cord blood progenitors were transduced with lentiviral luciferase GFP together with JAK2 WT-, MT-, and backbone lentiviral vectors, followed by intrahepatic transplantation into RAG2−/−γc−/− mice according to previously published methodology, and analyzed for human engraftment by noninvasive bioluminescent imaging and FACS (Traggiai et al., 2004). Transplanted RAG2−/−γc−/− mice were also treated with a selective JAK2 inhibitor (Fedratinib (TG-101348), 120 mg/kg) or vehicle (DMSO) by oral gavage twice daily for 12 days, and the effect on engraftment was analyzed. In another series of experiments, HSC were transduced with the JAK2V617F or backbone lentiviral vector with (+) or without (−) TG101348 (IN) or the vehicle (DMSO) and grown for 7 days in myelocult media, and transcript levels of erythroid transcription factors were quantified by Q-PCR (Jamieson et al., 2006). JAK2V617F-induced PV mouse model protocol: 1. Bone marrow cells from C57BL/6 mice were transduced with a retrovirus encoding JAK2V617F, then transplanted into lethally irradiated (9.5 Gy) recipient C57BL/6 mice (male, 8–10 weeks old). 2. Four weeks post-transplantation (when PV symptoms developed: Hct > 60%), mice were randomized into 3 groups (n=6/group): - Vehicle: 0.5% methylcellulose in PBS, oral gavage, daily; - Fedratinib (SAR302503) 30 mg/kg: dissolved in 0.5% methylcellulose, oral gavage, daily; - Fedratinib (SAR302503) 60 mg/kg: same solvent and route as 30 mg/kg group. 3. Treatment lasted 28 days. Blood samples were collected weekly to measure Hct and WBC count. 4. At euthanasia, spleens were weighed, and bone marrow/spleen tissues were fixed in 10% formalin for histopathological analysis [1] |
| 药代性质 (ADME/PK) |
Pharmacokinetic Properties of TG101348 in C57Bl/6 Mice [1]
The pharmacokinetic parameters of Fedratinib (TG-101348) were evaluated in C57Bl/6 mice following single oral administration of doses ranging from 30 mg/kg to 200 mg/kg. Maximum plasma concentrations (Cmax) of 0.68, 3.58, and 4.28 μM were observed at 3 hr postdose following oral gavage of 30, 100, and 200 mg/kg, respectively (Figure 1D). Following oral administration of TG101348, the total plasma exposure (AUC) increased linearly with respect to dose. At 7 and 24 hr postdose, the mean plasma concentrations were 0.483 and 0.02 μM for a 100 mg/kg dose, indicating that sustained plasma concentrations above the cellular IC50 could be achieved with twice daily (bid) administration. The steady-state plasma concentrations following bid administration showed no appreciable plasma accumulation. Based on the linearity and predictability of the TG101348 oral pharmacokinetics over dose range of 30 to 200 mg/kg, bid doses of 60 and 120 mg/kg were selected for evaluation in the murine model of polycythemia vera. Absorption, Distribution and Excretion A 400mg oral dose results in a Cmax of 1804ng/mL and an AUC of 26,870ng/*hr/mL. Fedratinib has a Tmax of 1.75-3 hours. A high fat breakfast does not significantly affect the absorption of fedratinib. An oral dose of fedratinib is 77% eliminated in the feces with 23% as unchanged drug. 5% is eliminated in the urine, with 3% as unchanged drug. The apparent volume of distribution is 1770L. The clearance of fedratinib is 13L/h. Metabolism / Metabolites Fedratinib is metabolized by CYP3A4, CYP2C19, and flavin-containing monooxygenase 3. Beyond that, data regarding the metabolism of fedratinib is not readily available. Biological Half-Life The half life of fedratinib is 41 hours with a terminal half life of 114 hours. Oral bioavailability in mice: Male C57BL/6 mice (8–10 weeks old) received Fedratinib (SAR302503) via oral gavage (10 mg/kg) or intravenous injection (2 mg/kg): - Oral bioavailability = 55%; - Oral administration: Cmax = 3.2 μg/mL (Tmax = 1.0 h), terminal half-life (t1/2) = 3.8 h, AUC0-24h = 14.5 μg·h/mL; - Intravenous administration: Cmax = 7.8 μg/mL, t1/2 = 3.5 h, AUC0-∞ = 26.4 μg·h/mL [1] - Plasma protein binding: In human plasma, Fedratinib (SAR302503) had a protein binding rate of 92%, primarily to albumin (measured by equilibrium dialysis at 37°C) [1] - Tissue distribution in PV mice: Oral Fedratinib (SAR302503) (60 mg/kg) resulted in bone marrow concentrations of 4.1 μg/g and spleen concentrations of 3.8 μg/g at 2 h post-administration, ~1.3-fold higher than plasma concentrations (3.2 μg/mL) [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In the prelicensure clinical trials of fedratinib in patients with myelofibrosis, liver test abnormalities were common but also found in a proportion of patients treated with placebo or with a comparator drug. Some degree of ALT elevation arose in up to 58% of fedratinib treated patients, compared to 14% to 17% of those treated with placebo, but were above 5 times the upper limit of normal (ULN) in 9% or less and were usually not accompanied by symptoms or jaundice. Nevertheless, at least one case of severe acute hepatitis with hepatic failure was reported in an early study of fedratinib. Subsequently, with more careful monitoring, instances of clinically apparent liver injury were not reported. Clinical experience with fedratinib since its approval has been limited. In addition, long term treatment with fedratinib and other Janus kinase inhibitors has been linked to rare instances of reactivation of hepatitis B that can be severe and even fatal. Reactivation often becomes clinically apparent after the JAK inhibitor is discontinued, when immune restoration results in an immunologic response to the heightened viral replication. Likelihood score: D (possible rare cause of clinically apparent liver injury including reactivation of hepatitis B in susceptible patients). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of fedratinib during breastfeeding. Most sources recommend that mothers not breastfeed while taking fedratinib. An alternate drug is preferred, especially while nursing a newborn or preterm infant. The manufacturer recommends that breastfeeding be withheld at least 1 month after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Fedratinib is ≥92% protein bound in plasma. n vitro normal cell safety: In normal human BMNCs (from healthy donors), Fedratinib (SAR302503) (≤1 μM) had no significant effect on BFU-E/CFU-E colony formation (viability >90% vs. vehicle) [2] - In vivo acute toxicity in PV mice: Fedratinib (SAR302503) (up to 60 mg/kg, oral, 28 days) caused no mortality or overt toxicity (e.g., lethargy, diarrhea). Platelet counts remained within normal range (250–500 × 10⁹/L) in all treatment groups [1] - No hepatotoxicity/nephrotoxicity: Serum ALT (52 ± 6 U/L vehicle vs. 48 ± 5 U/L 60 mg/kg) and creatinine (0.5 ± 0.1 mg/dL vehicle vs. 0.48 ± 0.1 mg/dL 60 mg/kg) were unchanged vs. vehicle [1] |
| 参考文献 | |
| 其他信息 |
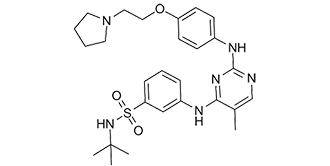
N-tert-butyl-3-[[5-methyl-2-[4-[2-(1-pyrrolidinyl)ethoxy]anilino]-4-pyrimidinyl]amino]benzenesulfonamide is a sulfonamide.
Fedratinib, also known as SAR302503 and TG101348, is a tyrosine kinase inhibitor used to treat intermediate-2 and high risk primary and secondary myelofibrosis. It is an anilinopyrimidine derivative. Fedratinib was granted FDA approval on August 16, 2019. Fedratinib is an oral selective inhibitor of Janus associated kinase 2 (JAK-2) and FMS-like tyrosine kinase 3 (FLT3) that is used in the therapy of intermediate or high-risk, primary or secondary myelofibrosis. Fedratinib has been associated with a high rate of serum enzyme elevations during therapy, but has been associated with only rare instances of clinically apparent acute liver injury. Fedratinib is an orally bioavailable, small-molecule, ATP-competitive inhibitor of Janus-associated kinase 2 (JAK2) and FMS-like tyrosine kinase 3 (FLT3; CD135; STK1; FLK2), with potential antineoplastic activity. Upon oral administration, fedratinib competes with wild-type JAK2 as well as mutated forms for ATP binding, which may result in inhibition of JAK2 activation, inhibition of the JAK-STAT signaling pathway, inhibition of tumor cell proliferation and induction of tumor cell apoptosis. JAK2 is the most commonly mutated gene in bcr-abl-negative myeloproliferative disorders (MPDs). In addition, fedratinib targets, binds to and inhibits the activity of FLT3. This inhibits uncontrolled FLT3 signaling and results in the inhibition of proliferation in tumor cells overexpressing FLT3. FLT3, a class III receptor tyrosine kinase (RTK), is overexpressed or mutated in most B-lineage neoplasms and in acute myeloid leukemias and plays a key role in tumor cell proliferation. See also: Fedratinib Hydrochloride (active moiety of). Drug Indication Fedratinib is indicated for the treatment of adult patients with intermediate-2 or high-risk primary or secondary (post-polycythemia vera or post-essential thrombocythemia) myelofibrosis. Inrebic is indicated for the treatment of disease-related splenomegaly or symptoms in adult patients with primary myelofibrosis, post polycythaemia vera myelofibrosis or post essential thrombocythaemia myelofibrosis who are Janus Associated Kinase (JAK) inhibitor naïve or have been treated with ruxolitinib. Mechanism of Action Fedratinib is an inhibitor of Janus Activated Kinase 2 (JAK2) and FMS-like tyrosine kinase 3. JAK2 is highly active in myeloproliferative neoplasms like myelofibrosis. Fedratinib's inhibition of JAK2 inhibits phosphorylation of signal transducer and activator of transcription (STAT) 3 and 5, which prevents cell division and induces apoptosis. Pharmacodynamics Fedratinib is a kinase inhibitor that inhibits cell division and induces apoptosis. Patients taking fedratinib may experience anemia, thrombocytopenia, gastrointestinal toxicity, hepatic toxicity, or elevated amylase and lipase. These effects should be managed by reducing the dose, temporarily stopping the medication, or providing transfusions on a case by case basis. It was reported that TG101348, a selective small-molecule inhibitor of JAK2 with an in vitro IC50 of approximately 3 nM, shows therapeutic efficacy in a murine model of myeloproliferative disease induced by the JAK2V617F mutation. In treated animals, there was a statistically significant reduction in hematocrit and leukocyte count, a dose-dependent reduction/elimination of extramedullary hematopoiesis, and, at least in some instances, evidence for attenuation of myelofibrosis. There were no apparent toxicities and no effect on T cell number. In vivo responses were correlated with surrogate endpoints, including reduction/elimination of JAK2V617F disease burden assessed by quantitative genomic PCR, suppression of endogenous erythroid colony formation, and in vivo inhibition of JAK-STAT signal transduction as assessed by flow cytometric measurement of phosphorylated Stat5. [1] Polycythemia Vera (PV) is a myeloproliferative disorder (MPD) that is commonly characterized by mutant JAK2 (JAK2V617F) signaling, erythrocyte overproduction, and a propensity for thrombosis, progression to myelofibrosis, or acute leukemia. In this study, JAK2V617F expression by human hematopoietic progenitors promoted erythroid colony formation and erythroid engraftment in a bioluminescent xenogeneic immunocompromised mouse transplantation model. A selective JAK2 inhibitor, TG101348 (300 nM), significantly inhibited JAK2V617F+ progenitor-derived colony formation as well as engraftment (120 mg/kg) in xenogeneic transplantation studies. TG101348 treatment decreased GATA-1 expression, which is associated with erythroid-skewing of JAK2V617F+ progenitor differentiation, and inhibited STAT5 as well as GATA S310 phosphorylation. Thus, TG101348 may be an effective inhibitor of JAK2V617F+ MPDs in clinical trials. [2] Mechanism of action: Fedratinib (SAR302503) selectively inhibits JAK2 (including the oncogenic JAK2V617F mutant) by competing with ATP for binding to the kinase domain. This blocks JAK2-mediated STAT5 phosphorylation, suppressing downstream signaling pathways that drive proliferation and survival of JAK2-mutant hematopoietic cells in PV [1,2] - Therapeutic focus: Preclinical data supports Fedratinib (SAR302503) for the treatment of JAK2V617F-driven myeloproliferative neoplasms (MPNs), particularly polycythemia vera (PV), due to its high selectivity for JAK2 and minimal off-target effects on JAK1/JAK3 [1,2] - Drug development context: Fedratinib (SAR302503) (TG101348) was advanced as a clinical candidate for MPNs based on the preclinical efficacy in PV mouse models and selectivity for JAK2, addressing unmet needs in patients with JAK2-mutant MPNs [1] |
| 分子式 |
C27H36N6O3S
|
|---|---|
| 分子量 |
524.6781
|
| 精确质量 |
524.256
|
| 元素分析 |
C, 61.81; H, 6.92; N, 16.02; O, 9.15; S, 6.11
|
| CAS号 |
936091-26-8
|
| 相关CAS号 |
Fedratinib hydrochloride hydrate;1374744-69-0
|
| PubChem CID |
16722836
|
| 外观&性状 |
White to light yellow solid
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
713.7±70.0 °C at 760 mmHg
|
| 闪点 |
385.5±35.7 °C
|
| 蒸汽压 |
0.0±2.3 mmHg at 25°C
|
| 折射率 |
1.611
|
| LogP |
3.27
|
| tPSA |
120.09
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
11
|
| 重原子数目 |
37
|
| 分子复杂度/Complexity |
787
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=S(C1C=C(NC2C(C)=CN=C(NC3C=CC(OCCN4CCCC4)=CC=3)N=2)C=CC=1)(NC(C)(C)C)=O
|
| InChi Key |
JOOXLOJCABQBSG-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C27H36N6O3S/c1-20-19-28-26(30-21-10-12-23(13-11-21)36-17-16-33-14-5-6-15-33)31-25(20)29-22-8-7-9-24(18-22)37(34,35)32-27(2,3)4/h7-13,18-19,32H,5-6,14-17H2,1-4H3,(H2,28,29,30,31)
|
| 化学名 |
N-(tert-butyl)-3-((5-methyl-2-((4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)amino)pyrimidin-4-yl)amino)benzenesulfonamide
|
| 别名 |
Brand name Inrebic; SAR302503, TG101348; TG101348; TG 101348; TG-101348; SAR-302503; Fedratinib; 936091-26-8; Tg-101348; TG101348; N-(tert-butyl)-3-((5-methyl-2-((4-(2-(pyrrolidin-1-yl)ethoxy)phenyl)amino)pyrimidin-4-yl)amino)benzenesulfonamide; Inrebic; SAR-302503; SAR 302503
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 100 mg/mL (190.56mM)
Water: <1 mg/mL Ethanol: <1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.87 mg/mL (5.47 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.87 mg/mL (5.47 mM) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (3.96 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.08 mg/mL (3.96 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100μL 20.8mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: ≥ 2.08 mg/mL (3.96 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 6 中的溶解度: 1% DMSO+30% polyethylene glycol+1% Tween 80:30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9059 mL | 9.5296 mL | 19.0592 mL | |
| 5 mM | 0.3812 mL | 1.9059 mL | 3.8118 mL | |
| 10 mM | 0.1906 mL | 0.9530 mL | 1.9059 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05177211 | Recruiting | Drug: Fedratinib Pill | Myeloproliferative Neoplasm Chronic Neutrophilic Leukemia |
H. Lee Moffitt Cancer Center and Research Institute |
March 1, 2022 | Phase 2 |
| NCT05524857 | Recruiting | Drug: Fedratinib Oral Capsule 300 mg Drug: Decitabine 20 mg/m2 |
RMyeloproliferative Neoplasm | Joseph Jurcic | January 28, 2022 | Phase 1 |
| NCT04446650 | Active, not recruiting | Drug: Fedratinib | Primary Myelofibrosis | Celgene | October 12, 2020 | Phase 1 Phase 2 |
| NCT06073847 | Recruiting | Drug: Fedratinib | Primary Myelofibrosis Post-polycythemia Vera Myelofibrosis |
Bristol-Myers Squibb | July 13, 2023 |