| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
Gyrase ( IC50 = 1.25 μg/mL ); TOPO IV ( IC50 = 1.5-2.5 μg/mL ); Quinolone
|
|---|---|
| 体外研究 (In Vitro) |
Garenoxacin (BMS284756)(0-8 天)抑制支原体和解脲支原体测试菌株的生长,MIC90 ≤0.25 μg/mL[1]。
Garenoxacin (48 h) 的 MIC 为 0.0128-4.0 μg/mL,这可抑制野生型和突变型金黄色葡萄球菌[2]。 Garenoxacin 对拓扑异构酶 IV 的 IC50 为 1.25 至 2.5 μg/mL,对金黄色葡萄球菌促旋酶的 IC50 为 1.25 μg/mL。 Garenoxacin 具有从对环丙沙星敏感的金黄色葡萄球菌分离株中选择性富集氟喹诺酮类耐药突变体的趋势较低[3]。 对63株肺炎支原体、45株人型支原体、15株发酵支原体和68株脲原体进行了加雷沙星(BMS-284756)、去氟喹诺酮类药物及其他8种药物的体外敏感性测定。加兰诺沙星是活性最强的喹诺酮类药物,在<或=1 μ g/ml时抑制所有分离株。加列诺克星的MIC, 90%的分离株被抑制(MIC(90)s);<或=0.008 μ g/ml)比莫西沙星和克林霉素低至少4倍,比斯帕沙星低8倍,比左氧氟沙星和环丙沙星低64倍。加诺沙星MIC(90) <或=0.008 μ g/ml,比克林霉素和莫西沙星低4倍,比斯帕沙星低8倍,比左氧氟沙星和环丙沙星低64倍。所有15株发酵分枝杆菌分离株均被浓度<或=0.008微克/毫升的加林诺沙星抑制,使其成为对该菌最有效的药物。对于脲原体,加诺沙星MIC(90) (0.25 μ g/ml)与莫西沙星、多西环素相当,比左氧氟沙星、斯帕沙星低4倍,比阿奇霉素低8倍,比环丙沙星低32倍。加诺沙星和其他氟喹诺酮类药物通过最小杀菌活性测量和时间杀伤研究证明对肺炎支原体和人支原体具有杀菌活性。加兰诺沙星在治疗支原体和脲原体感染方面有很大的潜力,因此需要进一步的研究。[1] 研究人员通过遗传和生化研究确定了新型地氟喹诺酮类药物加雷沙星(BMS-284756, T-3811ME)在金黄色葡萄球菌中的靶酶相互作用。我们发现加诺沙星比环丙沙星对野生型金黄色葡萄球菌的活性高4到8倍。单个拓扑异构酶IV或回转酶突变仅导致加雷沙星MIC增加2- 4倍,而两个位点的突变组合导致MIC大幅增加(128倍)。诺拉外排泵的过表达对加诺沙星耐药的影响很小。加诺沙星为MIC的两倍时,耐药突变体(<7.4 × 10(-12)至4.0 × 10(-11))的选择比环丙沙星少5至6个对数单位。在单步突变中,拓扑异构酶IV或gyrase的喹诺酮耐药决定区(QRDR)内外的突变被选中,表明拓扑异构酶IV和gyrase具有双重靶向性。基因实验表明,其中三种新突变是产生耐药性的原因。对金黄色葡萄球菌中纯化的拓扑异构酶IV和gyrase的研究也表明,加兰诺沙星对拓扑异构酶IV和gyrase具有相似的活性(50%的抑制浓度,分别为1.25 ~ 2.5和1.25微g/ml),虽然其对拓扑异构酶IV的活性是环丙沙星的2倍,但对gyrase的活性是环丙沙星的10倍。本研究提供了第一个支持喹诺酮类药物双重靶向金黄色葡萄球菌拓扑异构酶IV和gyrase的遗传学和生化数据,并为qrdr扩展到grlB的5‘端和gyrA的3’端提供了遗传学证据。[2] 新的喹诺酮加雷沙星(BMS-284756)缺乏C-6氟,对其阻断金黄色葡萄球菌生长的能力进行了检测。MIC和突变体预防浓度(MPC)的测定显示,加诺沙星对多种环丙沙星敏感的分离株的效力是环丙沙星的20倍,其中一些对甲氧西林耐药。90%分离株的MPC(MPC(90))低于使用推荐剂量的加诺沙星所达到的公布的血清药物浓度。这些体外观察表明,在环丙沙星敏感的金黄色葡萄球菌分离株中,加诺沙星选择性富集氟喹诺酮耐药突变体的倾向较低。对于环丙沙星耐药菌株,90%的测试菌株被抑制的MIC低于血清药物浓度,而MPC(90)则没有。因此,对于这些菌株,加诺沙星浓度预计在大部分治疗时间内落在突变体选择窗口内(在MIC和MPC之间)。因此,加诺克星有望选择性地富集易感性更低的突变体。[3] |
| 体内研究 (In Vivo) |
Garenoxacin(12.5-50 mg/kg;皮下注射;一次)在肺炎链球菌感染的小鼠肺炎模型中对野生型菌株和携带单突变的突变体非常有效[4]。
Garenoxacin(10 和30 mg/kg;口服;一次)显着增加 BALB/c 雌性小鼠在由肺炎链球菌 D-979 引起的实验性继发性肺炎球菌肺炎中的存活时间[5],同时也减少了肺部活细胞的数量。 在肺炎链球菌感染的小鼠肺炎模型中,针对野生型菌株和携带单一突变的突变体,格尔德诺辛(12.5-50mg/kg;皮下注射;一次)表现出显着的疗效[4]。 当BALB/c雌性小鼠暴露于由肺炎链球菌 D-979 引起的实验性继发性肺炎球菌肺炎,给予加雷沙星(10 和 30 mg/kg;口服;一次)时,肺部活细胞计数减少,存活时间显着延长。 P-4241菌株感染小鼠经加列诺沙星或TVA (25 mg/kg体重)处理后的肺药动学参数如下:血清中最大药物浓度C(max);分别为17.3和21.2微g/ml), C(max)/MIC比分别为288和170,浓度-时间曲线下面积(AUC;48.5和250微克。h/ml), AUC/MIC比(分别为808和2000)。加诺沙星25和50 mg/kg对野生型菌株和携带单一突变的突变体非常有效(存活率为85%至100%)。TVA对这些菌株的治疗效果与加诺克星相同。TVA 200 mg/kg和加诺克星50 mg/kg对parC和gyrA双突变体和gyrA、parC和parE三突变体无效。只有当菌株发生喹诺酮类药物耐药的几个突变时,加诺克星的疗效才会降低。[4] 在流感病毒感染后的肺炎球菌肺炎小鼠模型中,加诺沙星比其他氟喹诺酮类药物更有效,并表现出高水平的肺部细菌根除,低死亡率和有效的组织病理学改善。加诺沙星可能用于治疗继发性肺炎球菌性肺炎后流感。[5] |
| 酶活实验 |
拓扑异构酶IV测定。[2]
十癸烯化试验的反应混合物(20 μl)含有50 mM Tris-HCl (pH 7.7)、5 mM MgCl2、5 mM二硫索糖醇、50 μg牛血清白蛋白/ ml 250 mM谷氨酸钾、1 mM ATP、100 ng动质体DNA和不同量的GrlA和GrlB。37℃孵育1 h后,加入EDTA至终浓度50 mM终止反应,产物在1%琼脂糖中电泳分析。凝胶电泳后用溴化乙锭染色。 DNA回转酶测定。[2] 在含有75 mM Tris-HCl (pH 7.5)、7.5 mM MgCl2、7.5 mM二硫苏糖醇、2mM ATP、75 μg牛血清白蛋白/ ml、30 mM KCl、250 mM谷氨酸钾和2 μg tRNA(以0.5 μg松弛的pBR322为底物)的缓冲液中测定DNA超卷活性,缓冲液的总容积为20 μl。反应在30°C下进行1 h,加入EDTA至终浓度为50 mM停止反应,产物在1%琼脂糖中进行电泳分析,用于拓扑异构酶IV检测。 |
| 细胞实验 |
细胞系:解脲支原体、肺炎支原体、发酵支原体和人支原体。
培养时间:人支原体 48 小时,解脲支原体 24 小时,肺炎支原体 4-8 天< br> 结果:对肺炎支原体、发酵支原体、人型支原体和解脲支原体菌株具有抑制作用。 MIC90 分别为 0.031 μg/mL、≤0.008 μg/mL、≤0.008 μg/mL 和 0.25 μg/mL。 药物敏感性测定。[2] 在含有连续两倍稀释抗生素的Trypticase大豆琼脂上至少重复两次测定mic,并在37°C孵育24和48 h后进行生长评分。用钠啶酸的mic筛选gyrA突变,用新生物素的mic筛选grlB突变,用溴化乙啶的mic筛选NorA过表达。在基因测试中,当遇到双重差异时,它们通过重复测试来证实。 突变体选择的频率。[2] 通过将适当稀释的金黄色葡萄球菌ISP794隔夜培养物在不加抗生素或加加诺克星或环丙沙星的情况下,以每种药物MIC的1倍、2倍、4倍和8倍的浓度,在脑心脏输注琼脂上镀上突变体。为了选择加诺克星,使用大的(150 × 15毫米)培养皿对1011 ~ 1012 CFU进行培养皿。每次电镀一式两份,至少重复两次。选择板37℃孵育。抗性突变体的选择频率以48 h时抗性菌落数与接种细胞数之比计算。选择的菌落在含有选定浓度的加诺沙星的脑心灌注琼脂板上传代一次,如有必要,在不含任何抗生素的脑心灌注琼脂上传代一次,然后在- 70°C的10%甘油中保存于脑心灌注肉汤中。 逐步选择抗药突变体。[2] 将金黄色葡萄球菌ISP794连续传代于含有加诺沙星浓度增加两倍的脑心灌注琼脂上,以确定可达到的最高耐药水平。从加诺克星治疗ISP794的MIC开始选择。在每一步中,将几个突变菌落传代于含有加诺克星选择浓度的脑心输注琼脂板上,然后在- 70°C保存并在高两倍抗生素浓度下传代。[2] 抗生素治疗[4] 使用野生型强毒青霉素敏感菌株(P-4241)和喹诺酮耐药突变体(parC、gyrA和外排单突变体以及parC和gyrA双突变体)攻击18小时后开始治疗。用parE和parC gyrA parE临床菌株攻毒后3小时开始治疗。加诺沙星和TVA分别以12.5、25和50 mg/kg的剂量皮下注射6次。TVA以50、100和200 mg/kg的剂量给具有双重突变的突变小鼠。感染的、未治疗的对照组小鼠接受相同体积的等渗盐水。每个治疗组15只动物。观察期为10 d。每天记录死亡率,并比较累积存活率。 |
| 动物实验 |
Animal Model: Swiss mice with S. pneumonia infection[4].
Dosage: 12.5, 25 and 50 mg/kg Administration: Subcutaneous injection, once Result: Significantly improved the survival rate. Bactericidal activity in vivo [4] The protocol used to study bactericidal activity in vivo was the same as that used for the mouse survival studies. The total CFU counts recovered from whole-lung homogenates were determined 6 h after the first treatment, which was initiated 18 h after bacterial challenge, and 12 h after the second, fourth, and sixth treatments at doses of 12.5 and 25 mg of garenoxacin per kg. Three mice were used for each dose and time point. Mice were killed by intraperitoneal injection of sodium pentobarbital and were exsanguinated by cardiac puncture; blood was used for culture. The lungs were removed and homogenized in 1 ml of normal saline. Serial 10-fold dilutions of the homogenates were plated on Columbia agar. Blood was cultured in brain heart infusion broth. After overnight culture, colonies were counted on agar plates seeded with lung samples, and blood cultures were examined for turbidity. Results are expressed as the mean ± standard deviation log10 CFU per lung and as the number of positive or negative blood cultures for groups of three mice each. Determination of garenoxacin concentrations in serum and lungs and PK analysis [4] Antibiotics were administered as a single s.c. dose of 25 mg of garenoxacin or TVA per kg to both infected and uninfected mice. Infected mice were treated at 18 h postinfection. Serum and lung samples were collected from groups of six mice at 0.25, 0.5, 1, 2, 4, 6, 8, 12, and 24 h after drug administration. All samples were stored at −20°C and protected from light to avoid garenoxacin degradation during analysis. Lung samples were crushed in liquid nitrogen with a magnetic crusher. Serum samples (100 μl) and lung tissue samples (20 to 50 mg of lung powder, as measured precisely) were prepared by mixing an internal standard with methanolic acid (100 and 500 μl, respectively). After precipitation or diffusion, vortexing or ultrasonic mixing, and centrifugation, 50 μl of the upper phase was injected into a high-performance liquid chromatographic system. The total drug concentration was determined by use of an octadecyl silyl column (Novapak C18; 4.6 by 150 mm) coupled to a spectrofluorometric detector operating at excitation and emission wavelengths of 280 and 415 nm, respectively. The mobile phase was a mixture of acetonitrile, sodium citrate buffer solution (pH 3.5), and water (22/15/63; vol/vol) with 0.2% triethylamine, adjusted to pH 4. The flow rate was 1.0 ml/min. The limits of quantification were 0.02 μg/ml and 0.05 μg/g for serum and lung tissue samples, respectively, and measurements were linear over the ranges of 0.2 to 10.0 μg/ml and 0.5 to 50.0 μg/g for serum and lung tissue samples, respectively. The coefficients of variation for quality control were below 10% for both serum and lung tissue samples. The pharmacokinetic (PK) parameters for TVA were evaluated as described elsewhere |
| 药代性质 (ADME/PK) |
The area under the unbound serum concentration-time curve over 24 h divided by the MIC (fAUC0–24/MIC) is one of the most important predictors for the clinical efficacy of fluoroquinolones (Craig, 1998). In this secondary pneumococcal pneumonia model following IAV infection, oral garenoxacin (10 and 30 mg/kg) had fAUC0–24/MIC ratios of 71.7 and 288 in the serum and fAUC0–24/MIC ratios of 106 and 381 in the lungs, resulting in effective bacterial eradication and excellent efficacy (Table 1). Although it is not clear yet whether the 3 quinolones show similar efficacy in the same fAUC0-24/MIC in the secondary pneumococcal pneumonia model or not, the fAUC/MIC90 ratio of garenoxacin at a clinical dose in human for S. pneumoniae is ≥352, which is also greater than those of levofloxacin (15.5) and moxifloxacin (107) (Chein et al., 1997, Takagi et al., 2008, Watanabe et al., 2012, Zeitlinger et al., 2003). It was considered that the potent antibacterial activity and favorable pharmacokinetic profile of garenoxacin reflected its excellent therapeutic effect on experimental secondary pneumococcal pneumonia following IAV infection. Although moxifloxacin (30 mg/kg) decreased the viable cells in the lung to a similar extent as garenoxacin despite of lower fAUC0-24/MIC than that of garenoxacin, its mortality of 40% was larger than that of garenoxacin (Table 1). Further study would be required for clarifying the difference in the target value of fAUC/MIC among the quinolones. [5]
|
| 参考文献 |
|
| 其他信息 |
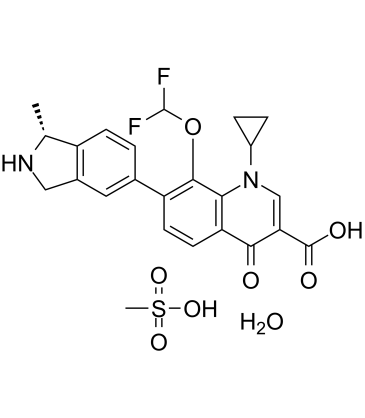
Garenoxacin is a quinolinemonocarboxylic acid that is 1,4-dihydroquinoline-3-carboxylic acid that is substituted by a cyclopropyl group at position 1, an oxo group at position 4, a (1R)-1-methyl-2,3-dihydro-1H-isoindol-5-yl group at position 7, and a difluoromethoxy group at position 8. It has a role as an antibacterial drug and a non-steroidal anti-inflammatory drug. It is a quinolone antibiotic, a quinolinemonocarboxylic acid, an organofluorine compound, a member of cyclopropanes, an aromatic ether and a member of isoindoles.
Garenoxacin, a quinolone antibiotic, is being investigated for the treatment of gram-positive and gram-negative bacterial infections. Drug Indication Investigated for use/treatment in bacterial infection. In addition to determining the MBCs for a subgroup of organisms, we sought to evaluate the dynamics of killing by garenoxacin of a representative isolate each of M. pneumoniae and M. hominis, since the MBCs had shown that garenoxacin had bactericidal effects against these organisms. Due to the slower growth rate of mycoplasmas, especially M. pneumoniae, with a generation time of 6 h, the usual 24-h duration of time-kill studies had to be lengthened in order to demonstrate an effect. We were able to demonstrate that garenoxacin has concentration-dependent bactericidal activity against M. pneumoniae after 24 to 96 h of incubation. Garenoxacin also demonstrated bactericidal activity against M. hominis after 24 h of incubation at four to eight times the MIC and after 48 h of incubation at two times the MIC. The regrowth of ≤2 log10 CFU observed in the presence of some of the lower concentrations of garenoxacin could have been due to a very small population of viable organisms that survived and that were allowed to propagate over time, perhaps aided by the degradation and inactivation of garenoxacin after prolonged incubation for several days in the case of M. pneumoniae. This is the first demonstration of the bactericidal effects of an antimicrobial agent against M. pneumoniae by time-kill studies modified from those commonly used to evaluate the effects of antimicrobials against other bacteria. The present study has shown that garenoxacin is a promising drug for the treatment of infections caused by Mycoplasma and Ureaplasma species. Further clinical evaluation should be pursued. [1] In summary, garenoxacin interacts similarly with both DNA gyrase and topoisomerase IV and has generated novel mutations expanding the range of the QRDR to both the amino-terminal domain of GrlB and the carboxy-terminal domain of GyrA. This novel desfluoroquinolone, with its high potency and low frequency of selection of resistant mutants, may thus prove advantageous in clinical settings, decreasing the possibility of selection of new resistant mutants. Strains with previously selected multiple quinolone resistance mutations, as is now common with methicillin-resistant clinical isolates of S. aureus, however, exhibit cross-resistance to garenoxacin that may limit the utility of this antibiotic for treatment of strains with established resistance to earlier quinolones. [2] For the isolates that were already resistant to ciprofloxacin, the MIC90 of garenoxacin was 3.2 μg/ml, roughly eight times higher than the MPC90 for the susceptible isolates. This observation suggests that multiple mutations were present in some of the resistant strains, as has been documented by other studies. Since the MIC for resistant isolates is below achievable serum drug concentrations (Fig. 2C), it is conceivable that treatment of ciprofloxacin-resistant S. aureus with garenoxacin might sometimes cure infection. However, the MPC90 of the isolates already resistant to ciprofloxacin was >19.6 μg/ml, which is well above the serum drug levels achieved with garenoxacin, even if daily doses are raised to 600 mg (Fig. 2C). Indeed, the MPC-based pharmacodynamics of garenoxacin and the mutants (Fig. 2C) are similar to those of ciprofloxacin and fully susceptible isolates (Fig. 2A). Since ciprofloxacin selected resistant mutants rapidly, we predict that additional mutations will be fixed in S. aureus if garenoxacin is used against ciprofloxacin-resistant strains. Those mutations would then preclude the use of garenoxacin in combination therapy. [3] In vivo, garenoxacin is as potent as TVA in terms of survival rates among mice infected with wild-type strains and resistant strains with single mutations and is slightly more effective than TVA against the mutants with double parC and gyrA mutations: 50 mg of garenoxacin per kg prolonged survival, whereas 200 mg of TVA per kg was ineffective. A comparison of the activity of garenoxacin with that of CIP, a well-characterized and widely distributed quinolone, showed that garenoxacin was far more effective. This was as expected, given the poor in vitro activity of CIP. The in vivo activity of garenoxacin is due to its better in vitro activity against wild-type and fluoroquinolone-resistant S. pneumoniae strains relative to that of CIP and its better activity against mutants with double and triple mutations compared to that of TVA. However, other factors, and particularly PK-PD parameters, are involved in the efficacies of quinolones in vivo. Forrest et al. and Hyatt et al. reported that the AUC/MIC ratio was the main parameter associated with bacterial eradication and clinical cure among patients with nosocomial pneumonia, with a minimal clinically effective ratio of 125. The favorable PK-PD parameters of garenoxacin thus contribute to its efficacy. Compared to CIP, garenoxacin has a longer half-life, larger AUCs, and superior in vitro activity, especially against S. pneumoniae; and garenoxacin yielded the highest AUC/MIC ratios in mouse serum and lung tissue samples. These PK and PD parameters are also very favorable for TVA, explaining why this quinolone is as effective as garenoxacin. Our pharmacokinetic data for garenoxacin closely match the mouse survival data, suggesting that serum protein binding has little influence on the therapeutic outcome, even though the level of serum protein binding reaches about 80% in mice (D. R. Andes and W. A. Craig, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-309, p. 10, 2003). This might be explained by the weak binding of garenoxacin to serum proteins. Moreover, inflammatory cells in lungs may serve as a reservoir, releasing garenoxacin in serum. TVA also shows high-level serum protein binding, while its efficacy is related to its good PK behavior. TVA was an interesting comparator in this mouse model of pneumococcal pneumonia, but it is clinically less relevant than garenoxacin because it has been withdrawn from the market. In conclusion, garenoxacin is highly effective in a mouse model of pneumonia induced by both quinolone-susceptible and quinolone-resistant strains of S. pneumoniae. Garenoxacin could thus be a useful option for the empirical treatment of community-acquired respiratory tract infections. [4] Previous studies have shown that β-lactam induced no improvement in survival from secondary bacterial pneumonia, despite effective bacterial eradication (McCullers, 2004), and that improved survival with macrolide-treatment was mediated by decreased inflammation (Karlström et al., 2009). Hara et al. (2011) has reported that garenoxacin has anti-inflammatory activity through its capacity to alter the secretion of interleukin 8 from both a human lung epithelial cell line and a human monocyte cell line, although in the case of lipopolysaccharide-stimulated cell. The improved outcome with garenoxacin might be related to not only bacterial eradication due to its greater fAUC0-24/MIC but also to the suppression of the inflammatory response. Thus, these data suggest a potential role for garenoxacin for the treatment of secondary pneumococcal pneumonia after influenza. Further studies would be required to better understand the influence of garenoxacin on inflammatory response and clinical efficacy in patients with secondary pneumococcal pneumonia. [5] |
| 分子式 |
C24H26F2N2O8S
|
|---|---|
| 分子量 |
540.53
|
| 精确质量 |
540.137
|
| 元素分析 |
C, 53.33; H, 4.85; F, 7.03; N, 5.18; O, 23.68; S, 5.93
|
| CAS号 |
223652-90-2
|
| 相关CAS号 |
194804-75-6; 223652-90-2 (mesylate hydrate); 223652-82-2 (mesylate)
|
| PubChem CID |
157690
|
| 外观&性状 |
Off-white to gray solid powder
|
| 沸点 |
581.5ºC at 760 mmHg
|
| 蒸汽压 |
2.29E-14mmHg at 25°C
|
| LogP |
5.316
|
| tPSA |
152.54
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
12
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
37
|
| 分子复杂度/Complexity |
863
|
| 定义原子立体中心数目 |
1
|
| SMILES |
O=C(C1=CN(C2CC2)C3=C(C=CC(C4=CC5=C([C@@H](C)NC5)C=C4)=C3OC(F)F)C1=O)O.CS(=O)(O)=O.O
|
| InChi Key |
IGTHEWGRXUAFKF-NVJADKKVSA-N
|
| InChi Code |
InChI=1S/C23H20F2N2O4.CH4O3S.H2O/c1-11-15-5-2-12(8-13(15)9-26-11)16-6-7-17-19(21(16)31-23(24)25)27(14-3-4-14)10-18(20(17)28)22(29)30;1-5(2,3)4;/h2,5-8,10-11,14,23,26H,3-4,9H2,1H3,(H,29,30);1H3,(H,2,3,4);1H2/t11-;;/m1../s1
|
| 化学名 |
1-cyclopropyl-8-(difluoromethoxy)-7-[(1R)-1-methyl-2,3-dihydro-1H-isoindol-5-yl]-4-oxoquinoline-3-carboxylic acid;methanesulfonic acid;hydrate
|
| 别名 |
BMS-284756-01; BMS284756-01; T-3811ME; 223652-90-2; Garenoxacin mesylate; Garenoxacin mesilate; Geninax; T3811ME; Garenoxacin mesylate; Garenoxacin mesylate hydrate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 2~100 mg/mL (4.7~185.0 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.63 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.63 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8500 mL | 9.2502 mL | 18.5004 mL | |
| 5 mM | 0.3700 mL | 1.8500 mL | 3.7001 mL | |
| 10 mM | 0.1850 mL | 0.9250 mL | 1.8500 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。