| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
DNA synthesis
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:吉西他滨诱导 BxPC-3、PANC-1 和 MIA PaCa-2 细胞中的 NF-κB 活性,并降低 BxPC-3 和 PANC-1 细胞中 NF-κB 抑制剂 IκBα 的水平。用低剂量吉西他滨处理 BxPC-3 细胞 48 小时会导致 NF-κB 结合呈剂量依赖性增加。相比之下,用较高吉西他滨剂量处理 48 小时的 BxPC-3 细胞中 NF-κB DNA 结合减少;然而,用这些较高剂量进行 24 小时处理会增加 BxPC-3 细胞中 NF-κB 的结合。细胞测定:将 BxPC-3、MIA PaCa-2 和 PANC-1 细胞接种到 96 孔板中。 24小时后,用媒介物、DMAPT和/或吉西他滨再处理细胞24小时或48小时。使用细胞死亡检测 ELISA 来定量细胞凋亡,以检测细胞质组蛋白相关 DNA 片段的量并相对于载体处理的细胞进行表达。
合成了一种新的嘧啶抗代谢药,2',2'-二氟脱氧胞苷,吉西他滨(LY188011,dFdCyd),并在实验性肿瘤模型中进行了评估。dFdCyd是一种非常有效和特异的脱氧胞苷类似物。在CCRF-CEM人白血病细胞培养试验中,50%抑制生长所需的浓度为1ng/ml。同时向细胞培养系统中添加脱氧胞苷会使生物活性降低约1000倍。[1] 在体外,细胞培养72小时,并暴露于药物1至72小时;通过多药效应分析评估协同作用。在野生型A2780和顺铂耐药的ADDP细胞中,同时暴露24和72小时是协同作用的,与顺铂预孵育4小时,然后是吉西他滨。在ADDP和A2780细胞中,用吉西他滨预孵育4小时,然后用吉西他滨和顺铂协同孵育。顺铂不会增强A2780和ADDP细胞中吉西他滨三磷酸的积累。顺铂导致吉西他滨引起的DNA双链断裂数量略有减少。[3] 吉西他滨是目前治疗癌症的最佳药物,但随着时间的推移,这种疾病会对药物产生耐药性。治疗癌症需要能够增强吉西他滨的作用或克服对该药物的化学耐药性的药物。姜黄素是姜黄(Curcuma longa)的一种成分,是一种已被证明可以抑制转录因子核因子κB(NF-kappaB)的药物,NF-kappbB与增殖、存活、血管生成和化疗耐药性有关。在本研究中,我们在体外和体内研究了姜黄素是否能使癌症对吉西他滨敏感。在体外,姜黄素抑制了各种胰腺癌症细胞系的增殖,增强了吉西他滨诱导的细胞凋亡,并抑制了细胞中组成型NF-κB的激活。[5] |
| 体内研究 (In Vivo) |
与 PBS 治疗的小鼠相比,吉西他滨治疗的小鼠瘤内 NF-κB 活性显着升高(1.3 至 1.8 倍),表明吉西他滨也诱导 NF-κB 激活。
抑制人白血病细胞在培养物中的生长导致该化合物作为潜在溶瘤剂的体内评估。每三天给药一次dFdCyd,体内活性最大。在本次评估中,将每天服用10天的1-β-D-阿拉伯呋喃糖胞嘧啶与dFdCyd进行了直接比较。dFdCyd在所评估的八种小鼠肿瘤模型中的八种中显示出良好至优异的抗肿瘤活性。在这些相同的肿瘤模型中,1-β-D-阿拉伯呋喃糖胞嘧啶的活性明显较低或没有活性。这种针对小鼠实体瘤的体内活性支持dFdCyd是治疗癌症的临床试验的优秀候选物的结论。[1] 在体内,最大耐受剂量为100或120mg/kg的吉西他滨可以与4mg/kg的顺铂联合使用。当同时注射时,这至少在HNX-22B中产生了额外的抗肿瘤活性,但在HNX-14C和结肠26-10肿瘤中没有。在吉西他滨之前或之后4小时注射顺铂,在HNX-22B肿瘤中与同步方案同样有效,但毒性更大。总之,吉西他滨和顺铂的组合在体外可以协同作用,在体内至少可以相加;这种协同作用取决于时间表。该机制不能通过吉西他滨三磷酸积累或DNA损伤研究来解释。[3] 在体内,与仅用橄榄油治疗的对照小鼠的肿瘤相比,注射胰腺癌症细胞并用姜黄素和吉西他滨组合治疗的裸鼠的肿瘤显示出显著的体积减少(与对照组相比P=0.008;与单独的吉西他宾相比P=0.036)、Ki-67增殖指数(与对照对照组比较P=0.030)、NF-kappaB活化和NF-kappaB-调节基因产物(细胞周期蛋白D1、c-myc、Bcl-2、Bcl-xL、细胞凋亡抑制剂蛋白-1、环氧化酶-2、基质金属蛋白酶和血管内皮生长因子)。联合治疗在抑制血管生成方面也非常有效,如CD31(+)微血管密度降低所示(与对照组相比P=0.018)。总之,我们的研究结果表明,姜黄素通过抑制增殖、血管生成、NF-κB和NF-κA调节的基因产物,增强吉西他滨在癌症中的抗肿瘤作用。[5] |
| 细胞实验 |
增殖试验。[5]
如前所述,姜黄素对细胞增殖的影响是通过3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化四唑(MTT)摄取法测定的。将细胞(每孔2000个)与姜黄素在96孔板中孵育三次,然后在37°C下孵育2、4或6天。向每个孔中加入MTT溶液,并在37°C下孵育2小时。加入提取缓冲液(20%SDS和50%二甲基甲酰胺),将细胞在37°C下孵育过夜。使用MRX Revelation 96孔多扫描仪在570nm处测量细胞悬浮液的吸光度。该实验重复两次,并进行统计分析(最初进行简单的线性回归分析,然后进行未配对的Student's t检验,显示两个样本均值之间存在显著差异)以获得最终值。[5] 细胞凋亡测定。[5] 为了确定姜黄素是否能增强吉西他滨对胰腺癌症细胞的凋亡作用,我们使用了活/死测定试剂盒,该试剂盒测定细胞内酯酶活性和质膜完整性。该检测使用钙黄绿素,一种保留在活细胞内的聚阴离子绿色荧光染料,以及一种红色荧光溴化乙锭同二聚体染料,它可以通过受损的膜进入细胞并与核酸结合,但被活细胞完整的质膜排除在外。简而言之,将细胞(每孔5000个)在室载玻片中孵育,用姜黄素预处理4小时,用吉西他滨处理24小时。然后在室温下用检测试剂染色30分钟。通过计数活(绿色)和死(红色)细胞,在荧光显微镜下测定细胞存活率。重复该实验两次,并进行统计分析。这些值最初进行了单因素方差分析,结果显示组间存在显著差异,然后使用未配对的Student t检验在组间进行了比较,结果显示两个样本均值之间存在显著差异。[5] 在 96 孔板中,接种 BxPC-3、MIA PaCa-2 和 PANC-1 细胞。 24小时后用媒介物、DMAPT和/或吉西他滨进一步处理细胞24或48小时。使用细胞死亡检测 ELISA,通过计算细胞质组蛋白相关 DNA 片段的数量来测量与载体处理的细胞相关的细胞凋亡。 |
| 动物实验 |
Female BALB/c nude mice
5 mg/kg i.p. After 1 week of implantation, mice were randomized into the following treatment groups (n = 6) based on the bioluminescence measured after the first IVIS imaging: (a) untreated control (olive oil, 100 μL daily); (b) curcumin alone (1 g/kg), once daily p.o.; (c) gemcitabine alone (25 mg/kg), twice weekly by i.p. injection; and (d) combination of curcumin (1 g/kg), once daily p.o., and gemcitabine (25 mg/kg), twice weekly by i.p. injection. Tumor volumes were monitored weekly by the bioluminescence IVIS Imaging System 200 using a cryogenically cooled imaging system coupled to a data acquisition computer running Living Image software. Before imaging, animals were anesthetized in an acrylic chamber with 2.5% isoflurane/air mixture and injected i.p. with 40 mg/mL d-luciferin potassium salt in PBS at a dose of 150 mg/kg body weight. After 10 min of incubation with luciferin, mice were placed in a right lateral decubitus position and a digital grayscale animal image was acquired followed by acquisition and overlay of a pseudocolor image representing the spatial distribution of detected photons emerging from active luciferase within the animal. Signal intensity was quantified as the sum of all detected photons within the region of interest per second. Mice were imaged on days 0, 7, 14, 21, 24, and 31 of treatment. Therapy was continued for 4 weeks and animals were sacrificed 1 week later. Primary tumors in the pancreas were excised and the final tumor volume was measured as V = 2 / 3πr3, where r is the mean of the three dimensions (length, width, and depth). The final tumor volumes were initially subjected to one-way ANOVA and then later compared among groups using unpaired Student's t test. Half of the tumor tissue was formalin fixed and paraffin embedded for immunohistochemistry and routine H&E staining. The other half was snap frozen in liquid nitrogen and stored at −80°C. H&E staining confirmed the presence of tumor(s) in each pancreas.[5] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Peak plasma concentrations of gemcitabine range from 10 to 40 mg/L following a 30-minute intravenous infusion, and are reached at 15 to 30 minutes. One study showed that steady-state concentrations of gemcitabine showed a linear relationship to dose over the dose range 53 to 1000 mg/m2. Gemcitabine triphosphate, the active metabolite of gemcitabine, can accumulate in circulating peripheral blood mononuclear cells. In one study, the Cmax of gemcitabine triphosphate in peripheral blood mononuclear cells occurred within 30 minutes of the end of the infusion period and increased increased proportionally with gemcitabine doses of up to 350 mg/m2. Gemcitabine mainly undergoes renal excretion. Within a week following administration of a single dose of 1000 mg/m2 infused over 30 minutes, about 92-98% of the dose was recovered in urine where 89% of the recovered dose was excreted as difluorodeoxyuridine (dFdU) and less than 10% as gemcitabine. Monophosphate, diphosphate, or triphosphate metabolites of gemcitabine are not detectable in urine. In a single-dose study, about 1% of the administered dose was recovered in the feces. In patients with various solid tumours, the volume of distribution increased with infusion length. The volume of distribution of gemcitabine was 50 L/m2 following infusions lasting less than 70 minutes. For long infusions, the volume of distribution rose to 370 L/m2. Gemcitabine triphosphate, the active metabolite of gemcitabine, accumulates and retains in solid tumour cells _in vitro_ and _in vivo_. It is not extensively distributed to tissues after short infusions that last less than 70 minutes. It is not known whether gemcitabine crosses the blood-brain barrier, but gemcitabine is widely distributed into tissues, including ascitic fluid. In rats, placental and lacteal transfer occurred rapidly at five to 15 minutes following drug administration. Following intravenous infusions lasting less than 70 minutes, clearance ranged from 41 to 92 L/h/m2 in males and ranged from 31 to 69 L/h/m2 in females. Clearance decreases with age. Females have about 30% lower clearance than male patients. Gemcitabine pharmacokinetics are linear and are described by a 2-compartment model. Population pharmacokinetic analyses of combined single and multiple dose studies showed that the volume of distribution of gemcitabine was significantly influenced by duration of infusion and gender. Clearance was affected by age and gender. Differences in either clearance or volume of distribution based on patient characteristics or the duration of infusion result in changes in half-life and plasma concentrations. Protein binding /of gemcitabine/ is very low, less than 10%. It is not known wether gemcitabine or its metabolites are distributed into breast milk. /Elimination is/ renal. 92 to 98% of a single dose of radiolabeled gemcitabine (1000 mg per square meter of body surface area, given over 30 minutes to five patients) was recovered within 1 week, primarily as the inactive uracil metabolite (approximately 89% of the excreted dose) and secondarily as unchanged gemcitabine (less than 10% of the excreted dose). For more Absorption, Distribution and Excretion (Complete) data for GEMCITABINE (8 total), please visit the HSDB record page. Metabolism / Metabolites Following administration and uptake into cancer cells, gemcitabine is initially phosphorylated by deoxycytidine kinase (dCK), and to a lower extent, the extra-mitochondrial thymidine kinase 2 to form gemcitabine monophosphate (dFdCMP). dFdCMP is subsequently phosphorylated by nucleoside kinases to form active metabolites, gemcitabine diphosphate (dFdCDP) and gemcitabine triphosphate (dFdCTP). Gemcitabine is also deaminated intracellularly and extracellularly by cytidine deaminase to its inactive metabolite 2′,2′-difluorodeoxyuridine or 2´-deoxy-2´,2´-difluorouridine (dFdU). Deamination occurs in the blood, liver, kidneys, and other tissues, and this metabolic pathway accounts for most of drug clearance. Gemcitabine undergoes intracellular metabolism, via nucleoside kinases, to produce two active metabolites (gemcitabine diphosphate and gemcitabine triphosphate) and also undergoes deamination to an active uracil metabolite. ... After intravenous injection, gemcitabine is rapidly converted to the inactive metabolite 2'-deoxy-2',2'-difluorouridine by cytidine deaminase. ... Biological Half-Life Following intravenous infusions lasting less than 70 minutes, the terminal half-life ranged from 0.7 to 1.6 hours. Following infusions ranging from 70 to 285 minutes, the terminal half-life ranged from 4.1 to 10.6 hours. Females tend to have longer half-lives than male patients. Gemcitabine triphosphate, the active metabolite of gemcitabine, can accumulate in circulating peripheral blood mononuclear cells. The terminal half-life of gemcitabine triphosphate, the active metabolite, from mononuclear cells ranges from 1.7 to 19.4 hours. The current study was performed in nonhuman primates to determine the plasma and CSF pharmacokinetics of gemcitabine and its inactive metabolite, difluorodeoxyuridine (dFdU) following iv administration. Gemcitabine, 200 mg/kg, was administered iv over 45 min to four nonhuman primates. Serial plasma and CSF samples were obtained prior to, during, and after completion of the infusion for determination of gemcitabine and dFdU concentrations. ... Plasma elimination was rapid with a mean t1/2 of 8 +/- 4 min (mean +/- SD) for gemcitabine and 83 +/- 8 min for dFdU. Gemcitabine total body clearance (ClTB) was 177 +/- 40 mL/min per kg and the Vdss was 5.5 +/- 1.0 L/kg. The maximum concentrations (Cmax) and areas under the time concentration curves (AUC) for gemcitabine and dFdU in plasma were 194 +/- 64 uM and 63.8 +/- 14.6 uM.hr, and 783 +/- 99 uM and 1725 +/- 186 uM.hr, respectively. The peak CSF concentrations of gemcitabine and dFdU were 2.5 +/- 1.4 uM and 32 +/- 41 uM, respectively. The mean CSF:plasma ratio was 6.7% for gemcitabine and 23.8% for dFdU. There is only modest penetration of gemcitabine into the CSF after iv administration. In this study, the plasma pharmacokinetics (PKs) of gemcitabine and dFdU are further explored after gemcitabine doses of 10, 30, and 60 mg/kg administered by intravenous infusion with a loading dose /to dogs/. Gemcitabine displayed linear PKs, while the kinetics of 2',2'-difluorodeoxyuridine (dFdU) were not dose proportional. The overall clearance, volume of distribution at steady-state, and terminal elimination half-life (t(1/2)) for gemcitabine were 0.421 L/hr.kg, 0.822 L/kg, and 1.49 hr, respectively. Plasma concentrations of dFdU peaked at approximately 2 hr postdosing and had a t(1/2) of 14.9 hr. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Elevations in serum aminotransferase levels occur in 30% to 90% of patients receiving cyclic therapy with gemcitabine. The elevations are generally mild-to-moderate, asymptomatic and self-limited, frequently resolving without discontinuation or even interruption of therapy. ALT or AST elevations above 5 times the upper limit of the normal range occur in 1-4% of patients yet rarely lead to symptoms or clinically apparent liver injury. Serum bilirubin and alkaline phosphatase elevations are less common, but also typically transient and mild. Despite wide use, gemcitabine has only rarely been implicated in rare cases of acute liver injury with jaundice, and most published cases have been reported in patients with underlying chronic liver disease or extensive hepatic metastases. The clinical features of hepatotoxicity from gemcitabine have not been well described. Most cases were marked by a progressive cholestasis and hepatic failure developing after several cycles of therapy in patients with preexisting chronic liver disease (hepatitis C, alcoholic liver disease) or significant hepatic metastases or local invasion. As with many antineoplastic agents and regimens, therapy with gemcitabine has also been associated rare cases of with reactivation of hepatitis B in persons with preexisting HBsAg in serum. At least one case of sinusoidal obstruction syndrome (veno-occlusive disease) has been reported with use of gemcitabine in a patient with underlying chronic hepatitis C who received no other antineoplastic agent. Likelihood score: C (probable rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Most sources consider breastfeeding to be contraindicated during maternal antineoplastic drug therapy. It might be possible to breastfeed safely during intermittent gemcitabine therapy with an appropriate period of breastfeeding abstinence; the manufacturer recommends an abstinence period of at least 1 week after the last dose. Chemotherapy may adversely affect the normal microbiome and chemical makeup of breastmilk. Women who receive chemotherapy during pregnancy are more likely to have difficulty nursing their infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk A telephone follow-up study was conducted on 74 women who received cancer chemotherapy at one center during the second or third trimester of pregnancy to determine if they were successful at breastfeeding postpartum. Only 34% of the women were able to exclusively breastfeed their infants, and 66% of the women reported experiencing breastfeeding difficulties. This was in comparison to a 91% breastfeeding success rate in 22 other mothers diagnosed during pregnancy, but not treated with chemotherapy. Other statistically significant correlations included: 1. mothers with breastfeeding difficulties had an average of 5.5 cycles of chemotherapy compared with 3.8 cycles among mothers who had no difficulties; and 2. mothers with breastfeeding difficulties received their first cycle of chemotherapy on average 3.4 weeks earlier in pregnancy. Of the 9 women who received a fluorouracil-containing regimen, 8 had breastfeeding difficulties. Protein Binding Gemcitabine plasma protein binding is less than 10%. Interactions ... /The authors/ present the first case of a nonlung cancer patient experiencing not only acne-like skin toxicity, but subsequently also severe interstitial lung disease during therapy with gemcitabine and erlotinib. Both therapeutic agents were suspected as a possible cause of this adverse event. An interaction between gemcitabine and erlotinib might have also contributed to the pathogenesis of this pulmonary toxicity. Treatment with high-dose steroids was, however, very effective in our patient and a complete recovery appeared within a few days. Thus, pulmonary side effects should be regarded carefully in pancreatic cancer patients receiving palliative therapy with gemcitabine and erlotinib. /The authors/ investigated the possible pharmacokinetic interactions of gemcitabine and oxaliplatin in patients with advanced solid tumors. Ten patients with advanced stage solid tumors were treated with gemcitabine (1500 mg/sq m) as a 30-min intravenous infusion on days 1 and 8, followed by oxaliplatin (130 mg/sq m) as a 4-hr intravenous infusion, on day 8 every 21 days. Pharmacokinetic data for 24 hr after dosing were obtained for both day 1 (gemcitabine without oxaliplatin coadministration) and day 8 (gemcitabine with oxaliplatin) during the first cycle of treatment. Gemcitabine levels in plasma were quantified using a reverse-phase high-performance liquid chromatography assay with ultraviolet detection, and total and ultrafiltrated platinum levels by flameless atomic absorption spectrophotometry with deuterium correction. All pharmacokinetic parameters of gemcitabine seemed to be unchanged when coadministered with oxaliplatin (day 8) compared with pharmacokinetic data of gemcitabine given as a single agent (day 1). The mean (maximum) concentration of gemcitabine on days 1 and 8 was 13.57 (+/-7.42) and 10.23 (+/-5.21) mg/L, respectively (P=0.28), and the mean half-life was 0.32 and 0.44 hr, respectively (P=0.40). Similarly, the P-values for AUC0-24 and the observed clearance were 0.61 and 0.30, respectively. Plasma total and free platinum levels were in agreement with other published data. Gemcitabine disposition appeared to be unaffected by oxaliplatin coadministration because no significant changes in pharmacokinetics between day 1 (gemcitabine without oxaliplatin coadministration) and day 8 (gemcitabine with oxaliplatin) were observed. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Antineoplastic Gemcitabine in combination with paclitaxel is indicated for the first-line treatment of patients with metastatic breast cancer after the failure of prior anthracycline-containing adjuvant chemotherapy, unless anthracyclines are clinically contraindicated. /Included in US product label/ Gemcitabine is indicated as first-line therapy for locally advanced (nonresectable stage II or III) or metastatic (stage IV) adenocarcinoma of the pancreas. It is also indicated as second-line therapy for patients who have previously been treated with fluorouracil. Treatment with gemcitabine is primarily palliative. /Included in US product label/ Gemcitabine is indicated in combination with cisplatin as a first-line therapy for inoperable, locally advanced (Stage IIIA or IIIB) or metastatic (Stage IV) non-small cell lung carcinoma. /Included in US product label/ For more Therapeutic Uses (Complete) data for GEMCITABINE (9 total), please visit the HSDB record page. Drug Warnings A complete blood cell count (CBC), including differential and platelets, should be performed prior to each dose of gemcitabine. If myelosuppression is detected, therapy should be modified or temporarily withheld according to the degree of hematologic toxicity. For patients with absolute granulocyte counts of at least 1000/cu m and platelet counts of at least 100,000/cu m, no adjustment in dosage is necessary. For those with absolute granulocyte counts of 500-999/cu m or platelet counts of 50,000-99,000/cu m, 75% of the full dose should be given weekly. If the absolute granulocyte count is less than 500/cu m or the platelet count is less than 50,000/cu m, the weekly dose should be withheld until the counts exceed these levels. The diagnosis of hemolytic-uremic syndrome should be considered and gemcitabine should be discontinued immediately in patients who develop anemia with evidence of microangiopathic hemolysis, elevation of serum bilirubin or LDH, reticulocytosis, and/or severe thrombocytopenia with or without evidence of renal failure (e.g., elevation of serum creatinine or BUN). Gemcitabine should be discontinued immediately and appropriate supportive care (e.g., diuretics, corticosteroids) provided promptly in patients who develop severe adverse pulmonary effects. The bone marrow depressant effects of gemcitabine may result in an increased incidence of microbial infection, delayed healing, and gingival bleeding. Dental work, whenever possible, should be completed prior to the initiation of therapy or deferred until blood counts have returned to normal. Patients should be instructed in proper hygiene during treatment, including caution in use of regular toothbrushes, dental floss, and toothpicks. FDA Pregnancy Risk Category: D /POSITIVE EVIDENCE OF RISK. Studies in humans, or investigational or post-marketing data, have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective./ Pharmacodynamics Gemcitabine is a nucleoside analog that mediates its antitumour effects by promoting apoptosis of malignant cells undergoing DNA synthesis. More specifically, it blocks the progression of cells through the G1/S-phase boundary. Gemcitabine demonstrated cytotoxic effects against a broad range of cancer cell lines _in vitro_. It displayed schedule-dependent antitumour activity in various animal models and xenografts from human non-small cell lung cancer (NSCLC) and pancreatic cancer. Therefore, the antineoplastic effects of gemcitabine are enhanced through prolonged infusion time rather than higher dosage. Gemcitabine inhibited the growth of human xenografts from carcinoma of the lung, pancreas, ovaries, head and neck, and breast. In mice, gemcitabine inhibited the growth of human tumour xenografts from the breast, colon, lung or pancreas by 69 to 99%. In clinical trials of advanced NSCLC, gemcitabine monotherapy produced objective response rates ranging from 18 to 26%, with a median duration of response ranging from 3.3 to 12.7 months. Overall median survival time was 6.2 to 12.3 months. The combined use of cisplatin and gemcitabine produced better objective response rates compared to monotherapy. In patients with advanced pancreatic cancer, objective response rates in patients ranged from 5.to 12%, with a median survival duration of 3.9 to 6.3 months. In Phase II trials involving patients with metastatic breast cancer, treatment with gemcitabine alone or with adjuvant chemotherapies resulted in response rate ranging from 13 to 42% and median survival duration ranging from 11.5 to 17.8 months. In metastatic bladder cancer, gemcitabine has a response rate 20 to 28%. In Phase II trials of advanced ovarian cancer, patients treated with gemcitabine had response rate of 57.1%, with progression free survival of 13.4 months and median survival of 24 months. Gemcitabine causes dose-limiting myelosuppression, such as anemia, leukopenia, neutropenia, and thrombocytopenia; however, events leading to discontinuation tend to occur less than 1% of the patients. Gemcitabine can elevate ALT, AST and alkaline phosphatase levels. |
| 分子式 |
C9H11F2N3O4
|
|
|---|---|---|
| 分子量 |
263.2
|
|
| 精确质量 |
263.071
|
|
| 元素分析 |
C, 41.07; H, 4.21; F, 14.44; N, 15.97; O, 24.31
|
|
| CAS号 |
95058-81-4
|
|
| 相关CAS号 |
|
|
| PubChem CID |
60750
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.8±0.1 g/cm3
|
|
| 沸点 |
468.0±55.0 °C at 760 mmHg
|
|
| 熔点 |
168.64°C
|
|
| 闪点 |
236.8±31.5 °C
|
|
| 蒸汽压 |
0.0±2.6 mmHg at 25°C
|
|
| 折射率 |
1.652
|
|
| LogP |
-1.24
|
|
| tPSA |
110.6
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
2
|
|
| 重原子数目 |
18
|
|
| 分子复杂度/Complexity |
426
|
|
| 定义原子立体中心数目 |
3
|
|
| SMILES |
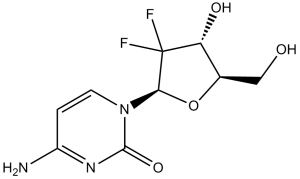
FC1([C@H](O)[C@@H](CO)O[C@H]1N1C=CC(N)=NC1=O)F
|
|
| InChi Key |
SDUQYLNIPVEERB-QPPQHZFASA-N
|
|
| InChi Code |
InChI=1S/C9H11F2N3O4/c10-9(11)6(16)4(3-15)18-7(9)14-2-1-5(12)13-8(14)17/h1-2,4,6-7,15-16H,3H2,(H2,12,13,17)/t4-,6-,7-/m1/s1
|
|
| 化学名 |
4-amino-1-[(2R,4R,5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.62 mg/mL (9.95 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.62 mg/mL (9.95 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.58 mg/mL (9.80 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 2.08 mg/mL (7.90 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清的DMSO储备液加入400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 5 中的溶解度: ≥ 2.08 mg/mL (7.90 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100μL 20.8mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 6 中的溶解度: ≥ 2.08 mg/mL (7.90 mM) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 配方 7 中的溶解度: ≥ 2.62 mg/mL (9.95 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 8 中的溶解度: 20 mg/mL (75.99 mM) in 0.5%HPMC + 1%Tween80 (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7994 mL | 18.9970 mL | 37.9939 mL | |
| 5 mM | 0.7599 mL | 3.7994 mL | 7.5988 mL | |
| 10 mM | 0.3799 mL | 1.8997 mL | 3.7994 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Study to Evaluate the Safety, Pharmacokinetics, and Activity of RO7496353 in Combination With a Checkpoint Inhibitor With or Without Standard-of-Care Chemotherapy in Participants With Locally Advanced or Metastatic Solid Tumors
CTID: NCT05867121
Phase: Phase 1 Status: Active, not recruiting
Date: 2024-12-02