| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
DPP4
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:Glimepiride格列美脲通过与两个位点相互作用抑制 Kir6.2/SUR 电流:Kir6.2 上的低亲和力位点 (IC(50)= 约 400 mM) 和 SUR 上的高亲和力位点 (IC(50) SUR1 = 3.0 nM,SUR2A = 5.4 nM,SUR2B = 7.3 nM)。与格列本脲相比,格列美脲在刺激正常脂肪细胞和胰岛素抵抗脂肪细胞和肌肉细胞中的葡萄糖转运、葡萄糖转运蛋白同工型 4 (GLUT4) 易位、脂质和糖原合成以及潜在的潜在信号传导过程方面表现出更高的效力在分子水平上进行检查。格列美脲以时间和浓度依赖性的不饱和方式与可能对应于小凹的质膜的去污剂不溶性复合物结合。格列美脲 (Glimepiride) 阻断吡那地尔激活的心肌细胞全细胞 K(ATP) 电流,IC(50) 为 6.8 nM,与格列本脲在这些细胞中的效力相当。格列美脲阻断 HEK 293 细胞中由外侧切除斑块中 Kir6.2/SUR2A 亚基共表达形成的 K(ATP) 通道,IC(50) 类似,为 6.2 nM。细胞测定:当在生理胰岛素剂量和格列美脲 (10 μM) 存在下培养细胞时,2-脱氧葡萄糖摄取增加至对照的 186%。在没有胰岛素的情况下,格列美脲也能增加 2-脱氧葡萄糖的摄取。同时,格列美脲将 GLUT1 和 GLUT4 的表达分别增加至对照的 164% 和 148%。这些结果表明格列美脲通过胰岛素非依赖性途径增加心脏葡萄糖摄取。
|
| 体内研究 (In Vivo) |
一种全新的磺酰脲类药物是格列美脲(Glimepiride)。静脉注射 Hoe 490 后,兔子的血糖水平降低了 2.5 倍,口服格列本脲 (HB 419) 后,血糖水平降低了 3.5 倍[1]。格列美脲(格列美脲)可降低细胞外 Aβ40 和 Aβ42 水平。格列美脲有望成为治疗糖尿病相关AD的良好药物[2]。与其他磺酰脲类药物相比,格列美脲通常可降低低血糖风险并减少体重增加。由于格列美脲(glimeperide)对缺血预处理没有负面影响,因此用于心血管疾病患者可能更安全[3]。
磺酰脲类药物是全球数百万人服用的一类抗糖尿病药物。啮齿动物已被广泛用于实验室研究磺酰脲类药物。在这里,我们报告了用磺酰脲类药物(Glimepiride/格列美脲)治疗小鼠的研究结果,以了解该药物如何影响葡萄糖稳态和耐受性。我们使用来自当地药店的格列美脲测试了格列美吡啶对空腹血糖、糖耐量和胰岛素分泌的影响。我们还研究了对胰高血糖素、糖异生和胰岛素敏感性的影响。出乎意料的是,小鼠接触格列美脲与空腹高血糖、葡萄糖不耐受和胰岛素减少有关。循环胰高血糖素水平或糖异生没有变化。这种效果是剂量依赖性的,在两周内生效,并在取出后三周内逆转。格列美脲在所有评估的菌株中都产生了相同的效果:四种野生型菌株,以及转基因Grn-/-和糖尿病db/db小鼠。我们的研究结果表明,在小鼠中使用格列美脲作为降糖药应谨慎进行,并可能对小鼠模型作为研究人类药典的替代品产生更广泛的影响。[4] 格列美脲/Glimepiride治疗会导致葡萄糖耐量受损[4] 为了尽量减少对动物的压力,我们选择在食物中服用Glimepiride/格列美脲。将野生型C57Bl/6J小鼠随意喂食格列美脲两周,然后进行葡萄糖耐量试验。格列美脲耐受良好,无明显不良并发症,包括未观察到低血糖事件。格列美脲治疗没有引起体重变化(未显示)。与已发表的报告相反,格列美脲治疗在葡萄糖注射后的大多数时间点都增加了空腹血糖和血糖(图1(a)),至少在8 mg/kg/天。随着时间的推移,曲线下面积也有所增加,表明葡萄糖耐量受损(图1(b))。较低剂量(1mg/kg/天)的曲线下面积呈增加趋势(p=0.07)。 |
| 酶活实验 |
β-分泌酶活性测定[2]
根据制造商的说明,使用β-分泌酶荧光测定试剂盒测量用或不用不同浓度的Glimepiride/格列美脲处理的细胞中存在的β-分泌酶类活性。简而言之,用PBS洗涤细胞两次,并向培养皿中加入60μl提取缓冲液。在冰上孵育5分钟后,将提取物在10000×g下离心5分钟。将50μl上清液与等体积的2×反应缓冲液和2μl底物混合。将平板在37°C的黑暗中保持90分钟,并使用微孔板读数器记录荧光。通过BCA法(Pierce)定量蛋白质浓度,并使用等量的细胞蛋白质来测量β-分泌酶活性。 γ-分泌酶无细胞测定[2] γ-分泌酶无细胞测定如前所述进行。简而言之,用15次杵A均质化大鼠皮质,通过离心(800×g,10分钟)分离核后组分。将上清液在4°C下以25000×g离心1小时,并将膜颗粒溶解在含有50 mM Tris-HCl、pH 6.8、2 mM EDTA、150 mM KCl和0.25%CHAPS的反应缓冲液中。在荧光测量之前,在有或没有格列美脲的情况下,将溶解膜(30μg)和γ-分泌酶荧光底物在37°C下孵育7小时。 |
| 细胞实验 |
Aβ40和Aβ42酶联免疫吸附试验(ELISA)[2]
为了测量细胞外Aβ40和Aβ42水平,从药物处理和未处理的细胞中收集条件培养基,在应用于ELISA板之前通过离心去除碎片。根据制造商的说明,分别使用人/大鼠Aβ40 ELISA试剂盒和人/大白鼠Aβ42 ELISA试剂盒对Aβ40和Aβ42水平进行定量。 蛋白质印迹 用PBS洗涤细胞,并在RIPA(50 mM Tris,pH 7.4,150 mM NaCl,1%NP-40,0.5%脱氧胆酸钠,0.1%SDS,补充有蛋白酶抑制剂混合物)中裂解。分别使用单克隆抗BACE1 C末端抗体(1:500)和单克隆抗β-肌动蛋白抗体(1:5000)通过蛋白质印迹分析定量细胞裂解物中BACE1和β-肌动蛋白的水平。然后使用标准ECL检测程序,并使用Quantity One成像系统测定所得条带的相对吸光度。 |
| 动物实验 |
Information about the mouse strains used, including age, length of treatment, and tests performed, is summarized in Table 1. All strains were obtained from the Jackson Labs (C57Bl/6J, C57Bl/6N, BalbC, and C3H) or in-house breeding colonies at the University of Kentucky (Grn−/− [10, 11] and db/db). db/db mice were on a hybrid C57Bl/6J/CD-1/129 background, described previously. Mice were group housed, fed and provided with water ad libitum, and maintained on a constant 12-hour light/dark cycle. Glimepiride was obtained by prescription and milled into chow (1 or 8 mg/kg/day). We based our estimate of Glimepiride dose on a 25 g mouse, and an average food consumption of 5 g per day. Nicorandil was administered in drinking water (15 mg/kg/day), based on an average of 5 mL of water consumed per day. Control mice were fed a control dietwith a consistent nutrient content and given control water with no additives. For the wash-out experiment, mice were tested three weeks after removal of Glimepiride chow. Mice were euthanized by CO2 asphyxiation, followed by decapitation, and the liver and serum frozen until use.[4]
|
| 药代性质 (ADME/PK) |
Absorption and Distribution
• Absorption: Orally administered drugs are 100% absorbed in the gastrointestinal tract, primarily in the upper segment of the small intestine, with a bioavailability of approximately 80%8. The time to peak plasma concentration (Cmax) is 2-3 hours • Protein Binding Rate: Exceeds 99.5%, indicating high plasma protein binding Metabolism and Excretion • Metabolic Pathway: Complete metabolism occurs via hepatic oxidative biotransformation, primarily yielding two metabolites: o Cyclohexyl hydroxymethyl derivative (M1): Retains about 1/3 of pharmacological activity o Carboxylated derivative (M2): Exhibits no hypoglycemic activity • Half-Life: Approximately 5 hours, but the duration of action can extend up to 24 hours Other Characteristics • Dosage Range: 1.0–8.0 mg/day, adjusted to the minimum effective dose based on blood glucose levels Tissue Distribution: Higher concentrations are observed in the liver, kidneys, and muscles Metabolism / Metabolites Glimepiride has known human metabolites that include Cyclohexylhydroymethylglimepiride. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Because no information is available on the use of glimepiride during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. Monitor breastfed infants for signs of hypoglycemia such as jitteriness, excessive sleepiness, poor feeding, seizures cyanosis, apnea, or hypothermia. If there is concern, monitoring of the breastfed infant's blood glucose is advisable during maternal therapy with glimepiride. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. 3476 man TDLo oral 28 ug/kg/2D-I BLOOD: HEMORRHAGE; BLOOD: THROMBOCYTOPENIA; SKIN AND APPENDAGES (SKIN): DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE Annals of Pharmacotherpy., 34(120), 2000 3476 rat LD oral >10 gm/kg LIVER: OTHER CHANGES Arzneimittel-Forschung. Drug Research., 43(547), 1993 [PMID:8328999] 3476 rat LD intraperitoneal >3950 mg/kg LIVER: OTHER CHANGES Arzneimittel-Forschung. Drug Research., 43(547), 1993 [PMID:8328999] 3476 rat LD50 unreported >10 gm/kg Diabetes Frontier., 3(565), 1992 3476 mouse LD50 unreported >10 gm/kg Diabetes Frontier., 3(565), 1992 |
| 参考文献 |
|
| 其他信息 |
Glimepiride is a sulfonamide, a N-acylurea and a N-sulfonylurea. It has a role as a hypoglycemic agent and an insulin secretagogue.
Glimepiride is a Sulfonylurea. See also: Glimepiride (annotation moved to). Numerous lines of evidence suggest a strong link between diabetes mellitus and Alzheimer's disease (AD). Impaired insulin signaling and insulin resistance occur not only in diabetes but also in the brain of AD. Recent evidence has indicated that peroxisome proliferator-activated receptor γ (PPARγ) agonists thiazolidinediones (TZDs) can decrease β-amyloid peptide (Aβ) deposition, which is the core component of senile plaques in AD, but the underlying mechanisms still remain unclear. In this study, we investigated whether glimepiride with PPARγ-stimulating activity, an oral anti-diabetic drug, has similar effects on Aβ production in primary cortical neurons. We demonstrated that glimepiride decreased extracellular Aβ40 and Aβ42 levels. The effect of glimepiride on reduction of Aβ40 generation was mediated by downregulation of β-site APP-cleaving enzyme 1 (BACE1) mRNA and protein expression, and by suppression of BACE1 activity. In addition, we found that high glucose condition enhanced Aβ40 production and glimepiride significantly decreased high glucose-induced Aβ40 production. Finally, a specific PPARγ antagonist GW9662 reversed glimepiride inhibitory effect on Aβ40 generation, suggesting a PPARγ-dependent mechanism may be involved. Our data indicated that glimepiride may serve as a promising drug for the treatment of AD associated with diabetes.[2] Type 2 diabetes mellitus is characterized by insulin resistance and progressive β cell failure; therefore, β cell secretagogues are useful for achieving sufficient glycemic control. Glimepiride is a second-generation sulfonylurea that stimulates pancreatic β cells to release insulin. Additionally, is has been shown to work via several extra pancreatic mechanisms. It is administered as monotherapy in patients with type 2 diabetes mellitus in whom glycemic control is not achieved by dietary and lifestyle modifications. It can also be combined with other antihyperglycemic agents, including metformin and insulin, in patients who are not adequately controlled by sulfonylureas alone. The effective dosage range is 1 to 8 mg/day; however, there is no significant difference between 4 and 8 mg/day, but it should be used with caution in the elderly and in patients with renal or hepatic disease. In clinical studies, glimepiride was generally associated with lower risk of hypoglycemia and less weight gain compared to other sulfonylureas. Glimepiride use may be safer in patients with cardiovascular disease because of its lack of detrimental effects on ischemic preconditioning. It is effective in reducing fasting plasma glucose, post-prandial glucose, and glycosylated hemoglobin levels and is a useful, cost-effective treatment option for managing type 2 diabetes mellitus.[3] Sulfonylureas are a class of antidiabetes medications prescribed to millions of individuals worldwide. Rodents have been used extensively to study sulfonylureas in the laboratory. Here, we report the results of studies treating mice with a sulfonylurea (glimepiride) in order to understand how the drug affects glucose homeostasis and tolerance. We tested the effect of glimepiride on fasting blood glucose, glucose tolerance, and insulin secretion, using glimepiride sourced from a local pharmacy. We also examined the effect on glucagon, gluconeogenesis, and insulin sensitivity. Unexpectedly, glimepiride exposure in mice was associated with fasting hyperglycemia, glucose intolerance, and decreased insulin. There was no change in circulating glucagon levels or gluconeogenesis. The effect was dose-dependent, took effect by two weeks, and was reversed within three weeks after removal. Glimepiride elicited the same effects in all strains evaluated: four wild-type strains, as well as the transgenic Grn−/− and diabetic db/db mice. Our findings suggest that the use of glimepiride as a hypoglycemic agent in mice should proceed with caution and may have broader implications about mouse models as a proxy to study the human pharmacopeia.[4] |
| 分子式 |
C24H34N4O5S
|
|---|---|
| 分子量 |
490.62
|
| 精确质量 |
490.224
|
| 元素分析 |
C, 58.75; H, 6.99; N, 11.42; O, 16.31; S, 6.54
|
| CAS号 |
93479-97-1
|
| 相关CAS号 |
Glimepiride-d5;1028809-90-6; Glimepiride-d4-1; 1131981-29-7; 119018-30-3 (urethane); 119018-29-0 (sulfonamide); 93479-97-1
|
| PubChem CID |
3476
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
677.0±65.0 °C at 760 mmHg
|
| 熔点 |
212.2-214.5 °C
|
| 闪点 |
363.2±34.3 °C
|
| 蒸汽压 |
0.0±2.2 mmHg at 25°C
|
| 折射率 |
1.628
|
| LogP |
4.17
|
| tPSA |
133.06
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
895
|
| 定义原子立体中心数目 |
0
|
| SMILES |
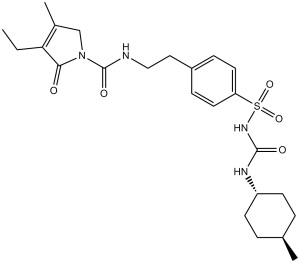
CCC1=C(CN(C1=O)C(=O)NCCC2=CC=C(C=C2)S(=O)(=O)NC(=O)NC3CCC(CC3)C)C
|
| InChi Key |
WIGIZIANZCJQQY-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C24H34N4O5S/c1-4-21-17(3)15-28(22(21)29)24(31)25-14-13-18-7-11-20(12-8-18)34(32,33)27-23(30)26-19-9-5-16(2)6-10-19/h7-8,11-12,16,19H,4-6,9-10,13-15H2,1-3H3,(H,25,31)(H2,26,27,30)
|
| 化学名 |
4-ethyl-3-methyl-N-[2-[4-[(4-methylcyclohexyl)carbamoylsulfamoyl]phenyl]ethyl]-5-oxo-2H-pyrrole-1-carboxamide
|
| 别名 |
HOE-490; Glimepiride; HOE 490; glimepiride; 93479-97-1; Amaryl; Glimepirida; Amarel; Glimepirid; Glimepiridum; Hoe-490; HOE-490; Amaryl; Glimepiridum; Amarel; Glimepirida; Roname
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (5.10 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.10 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0382 mL | 10.1912 mL | 20.3824 mL | |
| 5 mM | 0.4076 mL | 2.0382 mL | 4.0765 mL | |
| 10 mM | 0.2038 mL | 1.0191 mL | 2.0382 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Effect of Sodium-glucose Cotransporter-2 Inhibitor in Cellular Senescence in Patients With Cardiovascular Diseases or Type 2 Diabetes
CTID: NCT05975528
Phase: Phase 4 Status: Recruiting
Date: 2024-05-09
|
|---|
|