| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| Other Sizes |
|

| 靶点 |
Endogenous Metabolite; Microbial Metabolite
|
|---|---|
| 体外研究 (In Vitro) |
胆汁淤积症代表病理生理综合征,定义为肝脏胆汁流动受损。结果,胆汁酸积聚并促进肝细胞损伤,随后是肝硬化和肝功能衰竭。甘鹅脱氧胆酸(GCDCA)毒性相对较大,胆汁淤积后胆汁和血清中浓度较高。然而,GCDCA诱导肝毒性的机制尚不清楚。在这项研究中,我们发现GCDCA通过抑制溶酶体蛋白水解和增加溶酶体pH值来抑制自噬体形成并损害溶酶体功能,导致自噬清除缺陷,进而导致L02人肝细胞死亡。值得注意的是,通过基于串联质量标签(TMT)的定量蛋白质组学分析和数据库搜索,鉴定出313种差异表达的蛋白质,其中与对照组相比,GCDCA组有71种增加,242种减少。京都基因和基因组百科全书(KEGG)通路分析显示,GCDCA抑制了转录因子E3(TFE3)的信号通路,这与自噬通量损伤最密切相关。相比之下,GCDCA抑制溶酶体功能,TFE3过表达有效地减弱了自噬通量。具体而言,TFE3表达的降低与活性氧(ROS)稳态的破坏密切相关,这可以通过用N-乙酰半胱氨酸(NAC)抑制细胞内ROS来预防。总之,我们的研究首次证明,操纵ROS/TFE3信号传导可能是拮抗GCDCA诱导的肝毒性的一种治疗方法。[1]
方法:记录暴露于不同浓度有毒胆汁酸的胆管上皮细胞中TGF-βmRNA的表达;甘鹅脱氧胆酸(GCDCA)±PC。 结果:在这些实验中,以及在胆管上皮细胞与外周血单核细胞和肌成纤维细胞共同培养的共培养实验中,TGF-βmRNA的表达在PC存在或不存在的情况下保持不变。此外,肌成纤维组织的I型胶原α1 mRNA表达也保持不变。[2] 糖鹅脱氧胆酸/GCDC诱导的细胞凋亡对PKC活性的影响以及PKC在GCDCA诱导的肝细胞凋亡中的作用尚不清楚。本研究的具体目的是确定GCDC诱导的细胞凋亡是否会改变细胞内PKC活性,以及PKC活性的调节是否会影响GCDC引导的肝细胞凋亡。使用GCDC在分离的肝细胞中诱导凋亡。检测PKC活性,并使用特异性PKC和钙蛋白酶抑制剂研究PKC和钙粘蛋白调节对GCDC诱导的细胞凋亡的影响。暴露4小时后,50微M GCDC诱导42%的肝细胞凋亡。肝细胞暴露于GCDC 2小时后,细胞内PKC活性降至对照组的44%(p<0.001)。将肝细胞与钙蛋白酶抑制剂预孵育,使暴露于GCDC的肝细胞中的PKC活性恢复到91+/-5%的对照细胞。用钙蛋白酶抑制剂预孵育肝细胞可以减少GCDC诱导的凋亡,用激活PKC的佛波醇酯(PMA)预孵育也可以。钙蛋白酶抑制和PMA的联合使用进一步减少了GCDC诱导的细胞凋亡,但导致了低水平的肝细胞凋亡。白屈菜红碱抑制PKC也显著减少了GCDC诱导的肝细胞凋亡。GCDC诱导的细胞凋亡与总细胞PKC活性的降低有关,这似乎取决于细胞内钙蛋白酶样蛋白酶的活性。蛋白酶抑制和佛波酯预处理的组合保留了总细胞PKC活性,降低了GCDC诱导的凋亡,但在没有GCDC暴露的情况下诱导了低水平的凋亡。PKC抑制也降低了GCDC诱导的肝细胞凋亡,突显了PKC和蛋白酶在GCDC诱导凋亡过程中的复杂相互作用。[3] 据报道,胆汁酸(BA)的一种成分甘鹅脱氧胆酸(GCDC)可诱导原代人肝细胞坏死。在本研究中,我们探讨了GCDC在HCC化疗耐药性中的作用。我们发现GCDC通过分别下调和上调凋亡和抗凋亡基因的表达来促进HCC细胞的化疗耐药性。此外,GCDC诱导HCC细胞的EMT表型和干性,并激活STAT3信号通路。这些发现表明,GCDC通过STAT3途径诱导干性来促进HCC的化疗耐药性,并可能成为HCC化疗的潜在靶点[4]。 |
| 体内研究 (In Vivo) |
然后,我们评估了将这些选定的胆汁酸直接注射到脑室中是否也可以在体内抑制HPA轴。胆汁酸对HPA轴的抑制作用仅限于结合胆汁酸TCA和甘氨鹅脱氧胆酸(GCDA),而非结合胆汁酸CA、DCA和CDCA在注射后6小时对循环皮质酮水平没有显著影响(图3A)。同时,TCA和甘氨鹅脱氧胆酸(GCDA)注射液降低了下丘脑CRH mRNA表达和循环CRH蛋白水平(图3,B和C),而胆汁酸CA、DCA和CDCA没有显著影响(数据未显示)。[5]
为了评估胆汁酸的体内作用是否也依赖于ASBT,我们将ASBT vivo Morpholino或不匹配的对照序列注射到第三脑室。这显著抑制了下丘脑神经元中ASBT蛋白的翻译,如免疫荧光所示(图5A)。直接向第三脑室注射甘氨鹅脱氧胆酸(GCDA)显著抑制了下丘脑CRH mRNA表达(图5B)、蛋白质含量(图5C)和循环皮质酮水平(图5D),ASBT Vivo Morpholino注射可以减弱或逆转这一作用。[5] 然后,我们评估了胆汁酸激活GR的能力是否也与体内有关。大鼠侧脑室注射GR Vivo Morpholino(或不匹配的对照序列),通过免疫荧光评估下丘脑中GR的表达。正如预期的那样,通过免疫荧光(图7A)和免疫印迹(图7B)评估,与不匹配的对照组相比,中央GR Vivo Morpholino注射后GR免疫反应性受到显著抑制。此外,随后向第三脑室注射甘氨鹅脱氧胆酸(GCDA)显著抑制了CRH mRNA表达(图7C)、蛋白质含量(图7D)和循环皮质酮水平(图7E),这一作用在GR Vivo Morpholino注射后减弱。[5] |
| 酶活实验 |
GR萤光素酶测定[5]
此外,按照前面描述的程序,使用与含有糖皮质激素反应元件(GRE)共有序列的启动子区域偶联的萤光素酶报告构建体,在下丘脑神经元中评估GR转录活性。将神经元以10000个细胞/孔的密度铺在96孔板上,并让其粘附过夜。然后用GRE荧光素酶报告构建体(0.1-μg DNA/孔)和0.28μL TransIT-LT1转染试剂在37°C下转染细胞过夜。在此之后,用糖鹅脱氧胆酸(GCDA)或TCA(10μM)刺激细胞,并在刺激后24小时使用萤光素酶检测试剂盒检测萤光素酶活性。治疗至少进行了四次,结果以每微克蛋白质的萤光素酶活性变化程度表示。 |
| 细胞实验 |
细胞培养[1]
L02人正常肝细胞在1640培养基(HyClone)中培养,该培养基补充了10%热灭活FCS和1%(v/v)青霉素/链霉素(Sigma,St Louis,MO,USA),在37°C的5%CO2加湿气氛中。在80%融合时,细胞用不同浓度(50、75或100μM)的GCDCA处理6小时,或用100μM GCDCA处理不同时期(0、1、3或6小时),如我们之前的研究所述(Chen等人,20132015;Xu等人,2012)。将GCDCA溶解在无菌磷酸盐缓冲盐水(PBS)中以产生100mM储备溶液,然后在施用前用细胞培养基进行连续稀释。 细胞样本制备和胆汁酸检测[1] L02细胞(5×106个细胞)用100μMGCDCA处理6小时,细胞样品在冰上用500μl氯仿、甲醇和水(1:2.5:1,v/v/v)的混合物均质化。然后将样品在4°C下以13000 rpm离心10分钟,并将150-μl等分上清液转移到含有IS(10μl l-4-氯苯丙氨酸水溶液,5μg/mL)。用500μl甲醇对沉积物进行再均质化,并将150-μl等分上清液加入同一小瓶中干燥,然后用乙腈/H2O(6:4,v/v)复溶至最终体积为500μl。用流动相复溶后,用Waters ACQUITY超高效液相色谱法结合Waters XEVO TQ-S质谱仪和ESI源对提取物和胆汁酸参考标准品进行分析。整个UPLC-MS/MS系统由MassLynx 4.1软件控制。所有色谱分离均使用ACQUITY BEH C18柱(1.7μm,100 mm×2.1 mm内部尺寸),注射体积为5μL。使用TargetLynx应用程序管理器4.1版分析以阴性模式获得的UPLC-MS原始数据,以获得校准方程和样品中每种胆汁酸的定量浓度。使用Pierce BCA™蛋白质检测试剂盒测量的赖氨酸样品以ng/孔为单位进行测量,并按mg蛋白质进行标度。 细胞死亡试验[1] L02细胞被放置在6孔板中(每孔5×105个细胞)。用GCDCA处理后,用300μl胰蛋白酶-EDTA溶液分离细胞。将分离的细胞悬浮液在300g下离心5分钟。然后,将沉淀物与800μl台盼蓝溶液混合并分散。染色3分钟后,使用自动细胞计数器(Bio-Rad,TC10)对细胞进行计数。死细胞被染成蓝色。细胞死亡率(%)表示为死细胞/总细胞的百分比(Chang等人,2011)。 细胞培养[2] KMBC细胞在添加了110mg/L丙酮酸钠、10%胎牛血清(FBS)、100U/ml青霉素和100μg/ml链霉素的DMEM中培养。LX-2细胞在添加了0.1 mmol/L非必需氨基酸、2 mmol/L谷氨酰胺、110 mg/L丙酮酸钠、10%胎牛血清(FBS)、100 U/ml青霉素和100μg/ml链霉素的DMEM/F12中培养。所有细胞均在37°C、5%CO2和95%空气的加湿环境中孵育。在实验中,当细胞暴露于GCDCA时,暴露时间为24小时。 KMBC和LX-2细胞的共培养[2] KMBC和LX-2细胞在37°C下,在5%CO2和95%空气的加湿气氛中,在含有3.0um多孔膜的插入物的共培养板中培养。对于每个板,8×106 KMBC细胞在上室培养,8×10^6 LX-2细胞在下室培养。在处理前一天移除培养基,并在上述培养基中孵育细胞。在细胞收获前,将指示浓度的GCDCA加入上室浴溶液中24小时。 KMBC外周血单个核细胞(PBMC)和LX-2细胞的共培养[2] KMBC、PBMC(来源于健康供体)和LX-2细胞在37°C、5%CO2和95%空气的加湿气氛中,在含有3.0um多孔膜的插入物的共培养板中培养。对于每个孔,在上腔培养5×106 KMBC和3×106 PBMC细胞,在下腔培养8×106 LX-2细胞。在GCDCA暴露前一天移除培养基,并在上述培养基中孵育细胞。 细胞增殖和细胞毒性试验[4] 通过细胞计数试剂盒-8测定化疗诱导的细胞死亡。Huh7和LM3细胞以8×103个细胞/孔的密度接种在96孔板中,与GCDCA一起孵育,并分别用化疗药物(5-FU和顺铂)处理24小时和48小时。接下来,用磷酸缓冲盐水(PBS)洗涤细胞,并加入细胞计数试剂盒-8(CCK-8)溶液(培养基体积的1/10)1小时。使用微孔板读数器在450nm处检测细胞活力。 细胞凋亡测定[4] 将总共1×105个细胞接种在6孔板中,分别用GCDCA和化疗药物处理24小时和48小时。接下来,用PBS洗涤细胞,重新悬浮在PBS中,并根据制造商的说明用膜联蛋白V和碘化丙啶(PI)染色。流式细胞术用于分析凋亡细胞的比例。 |
| 动物实验 |
Male Sprague Dawley rats (150–175 g) were maintained in a temperature-controlled environment (20°C–22°C) with a 12-hour light, 12-hour dark cycle. Unless otherwise indicated, animals had free access to drinking water and standard rat chow. Rats were fed a diet containing 2% cholestyramine or the control diet AIN-93G for 3 days before either BDL or sham surgeries. Tissue and serum were collected 3 days after surgery between the hours of 8 and 9 am to minimize the circadian variations in glucocorticoid levels. In a separate experiment, rats were injected with 20 pmol of the bile acids cholic acid (CA), deoxycholic acid (DCA), chenodeoxycholic acid (CDCA), Glycochenodeoxycholic acid (GCDA), or TCA in the third ventricle (0 mm medial/lateral, −1.8 mm anterior/posterior, +4.5 mm dorsal/ventral) and serum and tissue were collected 6 hours later. In parallel, rats were infused with 1 mg/kg · d of Vivo-Morpholino sequences into the lateral ventricle at the coordinates (−1.3 mm medial/lateral, −0.2 mm anterior/posterior, +3.5 mm dorsal/ventral) using the brain infusion kits coupled to subcutaneous implanted minipumps for 3 days before the single Glycochenodeoxycholic acid (GCDA) or TCA injection following the method described above. The degree by which the target gene expression was suppressed by Vivo-Morpholino infusion was evaluated by immunofluorescence and immunoblotting as previously described [5].
|
| 参考文献 |
|
| 其他信息 |
Glycochenodeoxycholic acid is a bile acid glycine conjugate having 3alpha,7alpha-dihydroxy-5beta-cholan-24-oyl as the bile acid component. It has a role as a human metabolite. It is functionally related to a chenodeoxycholic acid. It is a conjugate acid of a glycochenodeoxycholate.
Glycochenodeoxycholic acid has been reported in Homo sapiens with data available. A bile salt formed in the liver from chenodeoxycholate and glycine, usually as the sodium salt. It acts as a detergent to solubilize fats for absorption and is itself absorbed. It is a cholagogue and choleretic. Recently, experimental evidence has indicated that deregulation of hepatic autophagic flux plays an important role in the pathogenesis of extrahepatic cholestasis. Impaired autophagy promoted bile acid-induced hepatic injury and the accumulation of ubiquitinated proteins, and activated of autophagy protected against cholestasis-induced hepatic injury (Gao et al., 2014; Kim et al., 2018). Moreover, Khambu B et al. reported that hepatic autophagy deficiency compromised farnesoid X receptor functionality and caused cholestatic injury (Khambu et al., 2019). Consistent with these findings, the results from our study confirmed that GCDCA inhibits autophagosome formation and impairs lysosomal function by inhibiting lysosomal proteolysis and increasing lysosomal pH, contributing to defects in autophagic clearance in vitro. The bHLH-leucine zipper protein TFE3, which belongs to the MiTF/TFE family, is a master regulator of autophagy and lysosomal biogenesis and stimulates the overall degradation of cells (Fan et al., 2018). TFE3 is emerging as a global regulator of cell survival and energy metabolism, both through the promotion of lysosomal genes and through newly characterized targets, such as oxidative metabolism and the oxidative stress response (Pi et al., 2019a; Wang et al., 2019). More recently, other MiTF/TFE proteins, namely, melanocyte inducing transcription factor (MITF), transcription factor EB (TFEB) and transcription factor EC (TFEC), major regulators of autophagy and lysosomal biogenesis, have emerged as leading factors in human disease pathology (Martina et al., 2014). In our research, GCDCA treatment inhibited TFE3 expression and suppressed TFE3 reporter activity, which decreased the expression of autophagy-related genes. MITF, TFEB, and TFE3 show a ubiquitous pattern of expression and have been detected in multiple cell types, whereas TFEC expression is restricted to cells of myeloid origin (Slade and Pulinilkunnil, 2017). On the basis of these results, we investigated whether the other MiT/TFE proteins are involved in the action of GCDCA in L02 cells. Consistent with the proteomic analysis, TFE3 mRNA expression decreased significantly after exposure to different concentrations of GCDCA for 6 h, and no significant changes were detected in the levels of MITF or TFEB (Fig. S6). These results confirm the important role of TFE3 in GCDCA-mediated autophagy. However, the mechanism that underlies the GCDCA-mediated inhibition of TFE3 expression and activity remains elusive. Recent studies have linked the accumulation of ROS to TFE3 activation in the invasion and migration of breast cancer or melanoma cells (Deng et al., 2018; Tan et al., 2018). ROS might play important roles in TFE3 inhibition in GCDCA-treated L02 cells. We found that GCDCA induced ROS generation in a dose-dependent manner, an effect abolished in cells pretreated with NAC. Furthermore, L02 cells incubated with NAC for 2 h prior to treatment with GCDCA showed inhibited ROS generation, which abrogated the effect of GCDCA on the TFE3 pathway. This result contradicts that of previous studies (Deng et al., 2018; Tan et al., 2018). We propose two possible reasons for this phenomenon. First, our results were obtained from a normal cell line, not a cancer cell line. Second, a very narrow spectrum of conditions was tested in the our study. The exact relationship between ROS and TFE3 may depend on the cell model, and the elucidation of the mechanistic details requires further research. In summary, our data suggest that ROS/TFE3 signaling may serve as a therapeutic target for the development of novel treatments to prevent liver damage in patients with extrahepatic cholestasis (Fig. 7). Notwithstanding the above findings, a number of limitations of the study warrant emphasis. First, only one cell line was used to evaluate the mechanism of GDCDA-induced hepatotoxicity, and other hepatic cell lines and/or primary hepatocytes will be studied in our future work. Second, only GCDCA and no other toxic bile acids were studied. Most importantly, our results are from cultured cells, and we should be careful extrapolating results from in vitro culture experiments to human patient populations. The problems of the current system are expected to be overcome by further improvements, including through the use of animal studies and rigorous clinical trials in our future work.[1] Notwithstanding the above findings, there are a number of limitations to the study that warrant emphasis. First, the cells employed included bile duct epithelial and myofibroblast cell lines rather than primary cells derived from human livers. Whether these cell lines are less sensitive to the toxic effects of GCDCA and pro-fibrogenic cytokine stimulation than primary cells remains to be determined. Second, only GCDCA and not other toxic bile acids were studied. Third, the concentration range of GCDCA was derived from previous reports documenting concentrations of GCDCA in human blood.14 Whether higher concentrations are present in human bile and in particular, the bile of PSC patients, is unclear. Fourth, the co-culture experiments physically separated bile duct epithelial and peripheral blood mononuclear cells from myofibroblasts thereby preventing cell–cell contact and possible intercellular communication. Fifth, perhaps longer periods of cell exposure to GCDCA were required to induce bile duct epithelial cell injury. However, the rapid biochemical and histologic changes associated with acute bile duct ligation models argue against that possibility.15 Sixth, it should be noted that PBMCs consist of peripheral blood monocytes and not tissue macrophages. Perhaps the additional features of the latter cell population are essential for the expression and release of pro-fibrogenic cytokines in this setting.16 Finally, we did not explore the possibility that restoring bile PC concentrations to normal levels may favorably alter the course of PSC by means other than protecting biliary tract epithelial cells from toxic bile acid-induced injury. In conclusion, the results of this study do not support the hypothesis that PC deficiency permits toxic bile acid-induced injury of biliary tract epithelial cells and subsequent activation of adjacent myofibroblasts, resulting in the enhanced fibrosis seen in PSC. Thus, at this time, restoration of low PC levels to normal values in PSC bile does not appear to be a worthwhile therapeutic approach to the treatment of PSC. [2] Studies have demonstrated that the JAK/STAT3 signaling pathway contributes to cell survival and chemotherapeutic resistance in cancers. Furthermore, it enhances the development of CSC-like characteristics. For example, the CSC marker, Nanog, is induced by the STAT3pathway in liver tumor-initiating cells. Similarly, members of the SOCS and PTPN families have been demonstrated to negatively affect the JAK/STAT signaling pathway. We found that GCDC activated the STAT3 signaling pathway by repressing the expression of several negative regulators of STAT3 signaling, including SOCS2, SOCS5, PTPN1, and PTPN11 in HCC cells. Glycochenodeoxycholic acid (GCDA)-induced resistance to drugs was inhibited when the expression of STAT3 was suppressed by siRNA in HCC cells. These results demonstrated that the STAT3 signaling pathway is involved in GCDC-induced chemoresistance of HCC cells. To summarize, our results showed that the treatment with GCDC enhanced the chemoresistance of HCC cells by inducing CSC-like characteristics and EMT phenotype, and activating the STAT3 signaling pathway via suppression of the expression of SOCS2, SOCS5, PTPN1, and PTPN1. Therefore, GCDC could serve as a potential target for the prognosis and therapy of HCC. [3] Suppression of the hypothalamic-pituitary-adrenal (HPA) axis has been shown to occur during cholestatic liver injury. Furthermore, we have demonstrated that in a model of cholestasis, serum bile acids gain entry into the brain via a leaky blood brain barrier and that hypothalamic bile acid content is increased. Therefore, the aim of the current study was to determine the effects of bile acid signaling on the HPA axis. The data presented show that HPA axis suppression during cholestatic liver injury, specifically circulating corticosterone levels and hypothalamic corticotropin releasing hormone (CRH) expression, can be attenuated by administration of the bile acid sequestrant cholestyramine. Secondly, treatment of hypothalamic neurons with various bile acids suppressed CRH expression and secretion in vitro. However, in vivo HPA axis suppression was only evident after the central injection of the bile acids taurocholic acid or Glycochenodeoxycholic acid (GCDA) but not the other bile acids studied. Furthermore, we demonstrate that taurocholic acid and Glycochenodeoxycholic acid (GCDA) are exerting their effects on hypothalamic CRH expression after their uptake through the apical sodium-dependent bile acid transporter and subsequent activation of the glucocorticoid receptor. Taken together with previous studies, our data support the hypothesis that during cholestatic liver injury, bile acids gain entry into the brain, are transported into neurons through the apical sodium-dependent bile acid transporter and can activate the glucocorticoid receptor to suppress the HPA axis. These data also lend themselves to the broader hypothesis that bile acids may act as central modulators of hypothalamic peptides that may be altered during liver disease.[5] |
| 分子式 |
C26H42NNAO5
|
|---|---|
| 分子量 |
471.6052
|
| 精确质量 |
471.296
|
| CAS号 |
16564-43-5
|
| 相关CAS号 |
Glycochenodeoxycholic acid;640-79-9;Glycochenodeoxycholic acid-d7 sodium;Glycochenodeoxycholic acid-d4;1201918-16-2
|
| PubChem CID |
12544
|
| 外观&性状 |
White to off-white solid powder
|
| 沸点 |
655.6ºC at 760mmHg
|
| 闪点 |
350.3ºC
|
| 蒸汽压 |
5.74E-20mmHg at 25°C
|
| LogP |
2.65
|
| tPSA |
109.69
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
32
|
| 分子复杂度/Complexity |
727
|
| 定义原子立体中心数目 |
10
|
| SMILES |
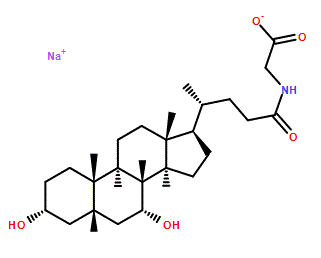
C[C@H](CCC(=O)NCC(=O)O)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2[C@@H](C[C@H]4[C@@]3(CC[C@H](C4)O)C)O)C
|
| InChi Key |
GHCZAUBVMUEKKP-GYPHWSFCSA-N
|
| InChi Code |
InChI=1S/C26H43NO5/c1-15(4-7-22(30)27-14-23(31)32)18-5-6-19-24-20(9-11-26(18,19)3)25(2)10-8-17(28)12-16(25)13-21(24)29/h15-21,24,28-29H,4-14H2,1-3H3,(H,27,30)(H,31,32)/t15-,16+,17-,18-,19+,20+,21-,24+,25+,26-/m1/s1
|
| 化学名 |
2-[[(4R)-4-[(3R,5S,7R,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoyl]amino]acetic acid
|
| 别名 |
16564-43-5; Glycochenodeoxycholic acid sodium salt; Sodium glycochenodeoxycholate; Sodium glycylchenodeoxycholate; NSC 681056; CHENYLGLYCINE SODIUM; Glycochenodeoxycholic acid (sodium salt); OK5NH65A9B;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~250 mg/mL (~530.10 mM)
H2O : ≥ 100 mg/mL (~212.04 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.41 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.41 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.41 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 50 mg/mL (106.02 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1204 mL | 10.6020 mL | 21.2040 mL | |
| 5 mM | 0.4241 mL | 2.1204 mL | 4.2408 mL | |
| 10 mM | 0.2120 mL | 1.0602 mL | 2.1204 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。