| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
CBP (IC50 = 0.02 μM); EP300 (IC50 = 0.03 μM); BRD4 (IC50 = 13 μM)[1]
|
|---|---|
| 体外研究 (In Vitro) |
GNE-272的选择性比BRD4高650倍,对CBP/EP300具有良好的选择性。 GNE-272 在 10 μM 浓度下对 42 个受体和 35 个激酶脱靶筛选进行测试,对任何靶标的抑制率均未超过 30%。此外,浓度大于 10 μM 的 GNE-272 不会抑制多种细胞色素 P450(3A4、1A2、2C9、2C19、2D6)。这种化学物质在BRET细胞测试中的功效良好。在靶点参与的正交评估中,发现 GNE-272 可降低 MYC10(MV4-11 细胞系)表达,EC50 为 0.91 μM,并且 BRET 和 MYC 细胞检测之间存在密切联系 [1]。
|
| 体内研究 (In Vivo) |
在小鼠 PK 测试中,GNE-272 以 100 mg/kg 剂量给药时,具有 26 μM 的良好口服生物利用度,但静脉注射 1 mg/kg 后显示出有限的清除率。不与 Cmax 一起使用。 GNE-272 影响体内 MYC 表达,并在血液癌细胞系中表现出强大的抗增殖作用,这两者都表明在 AML 肿瘤模型中具有抗肿瘤功效 [1]。
|
| 酶活实验 |
热位移测定方案[1]
他的Flag标记的人CBP溴结构域(K1082-G1197)在大肠杆菌中表达,并在内部纯化至>95%的纯度。在锥形管中,CBP(最终浓度为4μM)与SYPROOrange混合,在25 mM HEPES、100 mM NaCl、pH 6.8中的最终染料浓度为10倍。将试管短暂离心以去除沉淀物,然后将80μL蛋白质:染料溶液加入黑色透明底部384孔板的每个孔中,并短暂旋转(1分钟,900g)。从该储备板中,将23μL转移(每个储备板孔三次单独转移)到DMSO对照或从10 mM DMSO储备中镀入透明底Fluotrac200板的化合物中,使最终化合物浓度为50μM(1.0%v/v DMSO)。随后,将样品(15μL)转移到LightCycler 480板上,旋转(2分钟,900g),并在罗氏LightCycler 480 II上使用25-65°C的温度梯度和4.2°C/min的扫描速率进行分析。使用内部开发的应用程序评估熔融转变的中点(Tm),该应用程序测量荧光变化率随温度变化的一阶导数。相对于同一平板内的DMSO对照,计算化合物诱导的熔融温度变化ΔTm。 时间分辨荧光共振能量转移分析[1] 在一组生化溴结构域结合试验中评估了化合物的效力。通过时间分辨荧光共振能量转移(TR-FRET)评估生物素化小分子配体与重组His标记的溴结构域的结合。与生物素化配体竞争溴结构域结合的测试化合物会降低TR-FRET信号。所有生化测定方案均如前所述进行。 体外代谢稳定性实验[1] 用1μM化合物进行代谢稳定性实验,并使用先前报道的方法在雌性CD-1小鼠的混合肝微粒体和雌性CD-1鼠的混合冷冻肝细胞中进行评估。(25)肝微粒体孵育由0.5mg/mL微粒体蛋白、100mM Kpi缓冲液中的1mM NADPH组成。加入化合物(1μM)引发反应,在0、20、40和60分钟时取50μL等分试样,用2倍体积的含内标的乙腈淬灭反应。将冷冻保存的肝细胞解冻并重新悬浮在Dulbecco改良的Eagle培养基(DMEM,pH 7.4)中。将含有0.5×106个细胞/mL和1μM化合物的孵育混合物在37°C、95%相对湿度和5%CO2环境中孵育,在0、60、120和180分钟时取50μL等分试样,用含有内部内标的2倍体积乙腈淬灭反应。将来自微粒体和肝细胞的淬灭样品在2000g下离心10分钟。去除上清液并用水(2×)稀释,使用t=0峰面积比设置为100%通过LC-MS/MS进行分析。如Obach等人所述,确定了体外固有清除率和标度肝脏清除率。 体外蛋白质结合实验[1] 将小鼠血浆(100%)解冻,必要时用氢氧化钠或磷酸调节pH至7.4。将掺有化合物(终浓度5μM)的血浆(300μL)放入供体侧一次性RED板的样品室中,并将PBS的等分试样(500μL)放置在受体侧的相邻室中,两份均为两份。用透明的板盖覆盖平板,在37°C的利康振荡器上孵育4小时。4小时后,从受体和供体中取出40μL等分试样,加入含有2.5 nM普萘洛尔(内标)的乙腈(150μL)。为了创建分析上相同的样品基质以最小化基质效应,将40μL空白血浆添加到接收孔中,而将36μL空白等离子体和40μL PBS添加到供体孔中。样品在1000g下离心10分钟,上清液(100μL)转移到96孔分析板上。在LC-MS/MS分析之前,向样品中加入水(100μL)。蛋白质结合百分比由受体和供体侧检测到的分析物的面积比(归一化为内标)乘以100计算得出。 CYP抑制评估[1] 使用之前报道的方法,使用混合的(n=150)人肝微粒体,在0.16-10uM的GNE-272(化合物59)浓度范围内评估CYP抑制作用。孵育时间和蛋白质浓度取决于CYP亚型和评估的探针底物/代谢物。使用以下底物/代谢物和每种CYP的孵育时间和蛋白质浓度:CYP1A2,非那西丁/对乙酰氨基酚,30分钟,0.03 mg/mL蛋白质;CYP2C9,华法林/7-羟基华法林,30分钟,0.2 mg/mL蛋白质;CYP2C19,苯妥英/4-羟基苯妥英,40分钟,0.2 mg/mL蛋白质;CYP2D6,右美沙芬/右沙芬,10分钟,0.03 mg/mL蛋白质;CYP3A4,咪达唑仑/1-羟基咪达唑拉姆,10分钟,0.03 mg/mL蛋白质;CYP3A4睾酮/6β-羟基睾酮,10分钟,0.06mg/mL蛋白质。这些条件之前被确定为CYP特异性代谢物的线性形成速率。所有反应均用1 mM NADPH引发,并通过在含有适当稳定标记内标的乙腈中加入0.1%甲酸终止。样品通过LC-MS/MS进行分析。 |
| 细胞实验 |
细胞检测方案[1]
CBP BRET测定如前所述进行。为了确定对MYC分泌的抑制作用,将MV-4-11细胞(ATCC)以每孔10000个细胞的速度接种在96孔板上,培养基中添加了10%胎牛血清和2 mM l-谷氨酰胺。将稀释在DMSO中的试验化合物转移到细胞板上,使DMSO的最终浓度保持在0.1%,并在37°C下孵育4小时。使用QuantiGene 2.0试剂并按照供应商的说明进行MYC表达的裂解和分析。使用EnVision平板阅读器读取发光,并使用四参数非线性回归拟合在Genedata Screener中生成EC50s。 MDCK渗透率实验[1] 将MDCK细胞以2.5×105个细胞/mL的浓度接种在24孔板中,并在37°C、95%湿度和5%CO2的环境中生长4天。在接种后2天和实验前一天更换含有Dulbecco改良鹰培养基(DMEM)和补充有10%胎牛血清的Earle平衡盐溶液的培养基。将化合物以10μM的初始浓度添加到单层的顶端或基底外侧,并在37°C下孵育180分钟。在60、120和180分钟时从接收器室中取样,并通过LC-MS/MS进行分析。在顶端到基底外侧(A-B)和基底外侧到顶端(B-A)方向上的表观渗透率(Papp)计算为Papp=(dQ/dt)(1/AC0),其中dQ/dt=接收器室中化合物出现的速率,A=插入物的表面积,C0=时间=0时的初始底物浓度。流出比(ER)计算为(Papp,B-A/Papp,B-B)。 细胞中GNE-272 (compound 59)的体外评估[1] 人癌症细胞系在补充有10%胎牛血清和2mM谷氨酰胺的Dulbecco改良Eagle培养基中培养。培养物保持在37°C和80%湿度下。根据制造商的说明,使用RNeasy试剂盒从细胞中分离RNA。根据制造商的说明,使用iSCRIPT逆转录酶、TaqMan通用PCR混合物和基因特异性探针(ABI)通过RT-PCR测定MYC、其他癌基因和看家基因ACTB的RNA水平。使用抗MYC抗体通过蛋白质印迹法测定MYC蛋白水平。抗α-微管蛋白被用作负荷对照。使用HRP偶联的二抗检测信号。根据制造商的说明,使用CellTiter-Glo试剂在384孔板型中进行药物处理后评估细胞存活率。 |
| 动物实验 |
In Vivo PK of GNE-272 (compound 59)[1]
Six nu/nu mice were used. All animals were female, 6–9 weeks old at the time of study, and weighed between 15 and 25 g. Animals (n = 3 per dosing route) were dosed with GNE-272 (compound 59) 1 mg/kg iv (in propyl ethylene glycol 400 (35% v/v) and water (65% v/v)) or 100 mg/kg po (suspended in 0.5% w/v methylcellulose, 0.2% w/v Tween 80). Food and water were available ad libitum to all animals. Serial blood samples (15 μL) were collected by tail nick at 0.033, 0.083, 0.25, 0.5, 1, 3, 8, and 24 h after the intravenous administration and 0.083, 0.25, 0.5, 1, 3, 8, and 24 h after the oral administration. All blood samples were diluted with 60 μL of water containing 1.7 mg/mL EDTA and kept at −80 °C until analysis. Concentrations of GNE-272 (compound 59) were determined by a nonvalidated LC-MS/MS assay. The diluted blood samples were prepared for analysis by placing a 20 μL aliquot into a 96-well plate followed by the addition of 200 μL of acetonitrile containing an internal standard mixture (0.1 μg/mL diclofenac). The samples were vortexed and centrifuged at 4000 rpm for 20 min at 4 °C; 70 μL of the supernatant was diluted with 140 μL of 0.1% formic acid (FA) in water, and 10 μL of the solution was injected onto an analytical column. An ACQUITY UPLC system (Waters) coupled with an API 4000 mass spectrometer was used for sample analysis. The mobile phases were 0.3% FA and 2 mM NH4OAc in water/ACN (v:v, 95:5) (A) and 0.3% FA and 5 mM NH4OAc in ACN/water (v:v, 95:5) (B). The gradient was as follows: starting at 20% B and increased to 90% B for 1.2 min, maintained at 90% B for 0.4 min, then decreased to 20% B within 0.1 min. The total flow rate was 0.55 mL/min, and samples were injected onto an ACQUITY BEH C8 (100 mm × 2.1 mm, 1.7 μm) analytical column with a total run time of 1.7 min. Data were acquired using multiple reactions monitoring (MRM) in positive ion electrospray mode with an operating source temperature of 550 °C. The MRM transition was m/z 425.3 → 313.4 for GNE-272 (compound 59) and 296.0 → 214.0 for diclofenac. The lower and upper limits of quantitation of the assay for GNE-272 (compound 59) were 0.005 and 10 μM, respectively. In Vivo Evaluation of GNE-272 (compound 59) in MOLM-16 AML PK/PD and Antitumor Efficacy Model[1] RT-PCR was performed using TaqMan RNA-to-Ct 1-Step kit and Taqman Gene Expression Assays. The comparative Ct method was used to estimate relative changes in gene expression using MYC Taqman assay (Hs00153408_m1) and ACTB TaqMan assay (Hs01060665_g1) as housekeeping gene. All procedures were approved by and conformed to the guidelines and principles set by the Institutional Animal Care and Use Committee of Genentech and were carried out in an Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited facility. Female C.B-17 SCID.bg mice that were 8–9 weeks old and weighed 20–24 g were obtained from Charles River Lab. They were inoculated with five million MOLM-16 leukemia acute myelogenic cells (suspended in a 1:1 mixture of Hank’s Balanced Salt Solution containing Matrigel at a 1:1 ratio) in the right flank subcutaneously. Tumors were monitored until they reached a mean tumor volume of 130–300 mm3. The mean tumor volume across all eight groups was 222 ± 6.87 mm3 (mean ± SD) at the initiation of dosing. Mice were given 0 (vehicle, 0.5% methylcellulose; 0.2% Tween-80), 12.5, 25, and 50 mg/kg of compound GNE-272 (compound 59) by gavage, twice daily (BID) for 21 days in a volume of 100 μL. Tumor volumes were measured in two dimensions (length and width) using Ultra Cal-IV calipers and analyzed using Excel, version 11.2. The tumor volume was calculated with the following formula: tumor size (mm3) = (longer measurement × shorter measurement2) × 0.5. Animal body weights were measured using an Adventura Pro AV812 scale. Percent weight change was calculated using the following formula: group percent weight change = (new weight – initial weight)/initial weight) × 100. Plasma, tumor, and brain samples were collected at 2 h postdose. Concentrations of GNE-272 (compound 59) were determined by a nonvalidated LC-MS/MS assay. The plasma samples were prepared for analysis by placing a 25 μL aliquot into a 96-well plate. The tumor samples were collected and weighed. Four volumes of water by tissue weight were added. Using a bead beating homogenizer, the tissue samples were homogenized and 25 μL of each was aliquoted into a 96-well plate. A volume of 200 μL of acetonitrile containing an internal standard (labetalol) was added to the sample. The samples were vortexed and centrifuged at 4000 rpm for 10 min, and 50 μL of the supernatant was diluted with 150 μL of water. A 10 μL injection volume was used for analysis on a SIL-30ACMP autosampler system was linked to LC-30AD pumps, coupled with an API 5500 QTrap mass spectrometer was used for sample analysis. The mobile phases were 0.1% FA water (A) and 0.1% FA in ACN (B). The gradient was as following: starting at 10% B and increased to 90% B in 0.6 min, maintained at 90% B for 0.2 min, then decreased to 10% B within 0.1 min. The total flow rate was 1.2 mL/min and column for separation was Kinetex XB-C18 column (50 mm × 2.1 mm, 2.6 μm) with a total run time of 1.2 min. Data were acquired using multiple reactions monitoring (MRM) in positive ion electrospray mode with an operating source temperature of 550 °C. The MRM transition was m/z 425.3 → 313.4 for GNE-272 (compound 59) and 329.076 → 294.1 for labetalol. The lower and upper limits of quantitation of the assay for GNE-272 (compound 59) were 0.002 and 10 μM, respectively. |
| 参考文献 | |
| 其他信息 |
The single bromodomain of the closely related transcriptional regulators CBP/EP300 is a target of much recent interest in cancer and immune system regulation. A co-crystal structure of a ligand-efficient screening hit and the CBP bromodomain guided initial design targeting the LPF shelf, ZA loop, and acetylated lysine binding regions. Structure-activity relationship studies allowed us to identify a more potent analogue. Optimization of permeability and microsomal stability and subsequent improvement of mouse hepatocyte stability afforded 59 (GNE-272, TR-FRET IC50 = 0.02 μM, BRET IC50 = 0.41 μM, BRD4(1) IC50 = 13 μM) that retained the best balance of cell potency, selectivity, and in vivo PK. Compound 59 showed a marked antiproliferative effect in hematologic cancer cell lines and modulates MYC expression in vivo that corresponds with antitumor activity in an AML tumor model.[1]
|
| 分子式 |
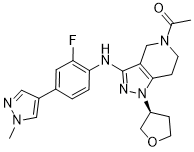
C22H25FN6O2
|
|---|---|
| 分子量 |
424.471307516098
|
| 精确质量 |
424.2023
|
| 元素分析 |
C, 62.25; H, 5.94; F, 4.48; N, 19.80; O, 7.54
|
| CAS号 |
1936428-93-1
|
| PubChem CID |
121372887
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
1.5
|
| tPSA |
77.2Ų
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
31
|
| 分子复杂度/Complexity |
656
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C(=O)(N1CC2C(NC3=CC=C(C4=CN(C)N=C4)C=C3F)=NN([C@H]3CCOC3)C=2CC1)C
|
| InChi Key |
NKOJNOBJGYTLLZ-KRWDZBQOSA-N
|
| InChi Code |
InChI=1S/C22H25FN6O2/c1-14(30)28-7-5-21-18(12-28)22(26-29(21)17-6-8-31-13-17)25-20-4-3-15(9-19(20)23)16-10-24-27(2)11-16/h3-4,9-11,17H,5-8,12-13H2,1-2H3,(H,25,26)/t17-/m0/s1
|
| 化学名 |
(S)-1-(3-((2-fluoro-4-(1-methyl-1H-pyrazol-4-yl)phenyl)amino)-1-(tetrahydrofuran-3-yl)-1,4,6,7-tetrahydro-5H-pyrazolo[4,3-c]pyridin-5-yl)ethan-1-one
|
| 别名 |
GNE-272; GNE 272; CHEMBL3897393; 1-[3-[[2-Fluoranyl-4-(1-Methylpyrazol-4-Yl)phenyl]amino]-1-[(3~{s})-Oxolan-3-Yl]-6,7-Dihydro-4~{h}-Pyrazolo[4,3-C]pyridin-5-Yl]ethanone; 1-[3-[2-fluoro-4-(1-methylpyrazol-4-yl)anilino]-1-[(3S)-oxolan-3-yl]-6,7-dihydro-4H-pyrazolo[4,3-c]pyridin-5-yl]ethanone; (S)-1-(3-((2-fluoro-4-(1-methyl-1h-pyrazol-4-yl)phenyl)amino)-1-(tetrahydrofuran-3-yl)-1,4,6,7-tetrahydro-5h-pyrazolo[4,3-c]pyridin-5-yl)ethan-1-one; 6XH; SCHEMBL17794706; GNE272.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~235.59 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.89 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.89 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.89 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3559 mL | 11.7794 mL | 23.5588 mL | |
| 5 mM | 0.4712 mL | 2.3559 mL | 4.7118 mL | |
| 10 mM | 0.2356 mL | 1.1779 mL | 2.3559 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。