| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
|
|
|---|---|---|
| 体外研究 (In Vitro) |
GNE-781 是一种高度发达、强效且特异性的 CBP(环磷酸腺苷反应元件结合蛋白)溴结构域抑制剂。 GNE-781 降低了 FOXP3(叉头盒 P3)的转录水平。对溴结构域子集的分析表明,GNE-781 对 CBP(5425 倍)和 P300(4250 倍)具有显着的选择性,并且对 CBP/P300 具有很高的选择性。 GNE-781 表现出理想比例的细胞效力和选择性 — 比 BRD4 高 5425 倍 (1) — [1]。
|
|
| 体内研究 (In Vivo) |
在携带 MOLM-16 AML 异种移植物的小鼠中,GNE-781(3-30 mg/kg;口服;每天两次,持续 21 天)在 3、10 和 30 mg/kg 剂量下抑制肿瘤生长抑制 (%TGI) 73% 、 71% 和 89% ,分别 [1]。 GNE-781 以剂量依赖性方式降低 Foxp3 转录水平。在剂量低至 3 mg/kg 时,GNE-781 (3-30 mg/kg) 在 2 小时和 8 小时抑制 MYC;在 10 和 30 mg/kg 浓度下,2 小时时达到最大抑制(87% 和 88% 抑制)[1]。
|
|
| 酶活实验 |
时间分辨荧光共振能量转移分析[1]
在一组生化溴结构域结合试验中评估了化合物的效力。通过时间分辨荧光共振能量转移(TR-FRET)评估生物素化小分子配体与重组His标记的溴结构域的结合。与生物素化配体竞争溴结构域结合的测试化合物会降低TR-FRET信号。所有生化测定方案均如前所述进行。 细胞检测方案[1] CBP BRET测定如前所述进行。为了确定MYC表达的抑制作用,将MV-4-11细胞(ATCC)以每孔10000个细胞的速度接种在96孔板中,培养基中补充有10%胎牛血清和2 mM l-谷氨酰胺。将稀释在DMSO中的试验化合物转移到细胞板上,使DMSO的最终浓度保持在0.1%,并在37°C下孵育4小时。使用QuantiGene 2.0试剂并按照供应商的说明进行MYC表达的裂解和分析。使用EnVision平板阅读器读取发光,并使用四参数非线性回归拟合在XLFit中生成EC50s。 |
|
| 细胞实验 |
化合物19(GNE-781)对Tregs的体外评价[1]
使用天然CD4+T细胞分离试剂盒II从健康供体的外周血单个核细胞中分离出人类天然CD4+T淋巴细胞,并在完全RPMI-1640培养基(10%FCS、50μM 2-巯基乙醇、10%青霉素/链霉素、10%NEAA、10%丙酮酸钠)中使用平板结合抗CD3(5μg/mL)、可溶性抗CD28(3μg/mL)加rTGFβ(5 ng/mL)和rIL-2(10 ng/mL。化合物19以2μM的浓度使用,并以2倍稀释度滴定 使用针对表面标记CD4 FITC(克隆OKT-4)和CD25 Pacific Blue(克隆BC96)的抗体对iTreg进行染色,用Foxp3/转录因子染色缓冲液组固定/渗透,并标记细胞内Foxp3 APC(克隆259D/C7)。使用可固定的活性染料efluor 781对iTreg进行活性染色。使用FACSDiva软件在BD LSR Fortessa上采集样本。使用FlowJo软件分析数据 使用RNeasy从iTregs中分离总RNA,包括柱上DNase I消化。使用高容量cDNA逆转录酶试剂盒制备cDNA。使用ABI 7900 HT快速实时PCR系统进行定量RT-PCR以确定Foxp3基因表达水平。基因表达数据被标准化为B2M作为管家基因。 |
|
| 动物实验 |
Mice[1] [1]
Twelve female CD-1 mice are used. All animals are 6-9 weeks old at the time of study and weighed between 20 and 35 g. Animals (n=3 per dosing route) are dosed with 10 or GNE-781 at 1 mg/kg iv (in propyl ethylene glycol 400 (35% v/v) and water (65% v/v)) or 5 mg/kg po (suspended in 0.5% w/v methylcellulose, 0.2% w/v Tween 80). Food and water are available ad libitum to all animals. Serial blood samples (15 μL) are collected by tail nick at 0.033, 0.083, 0.25, 0.5, 1, 3, 8, and 24 h after the intravenous administration and 0.083, 0.25, 0.5, 1, 3, 8, and 24 h after the oral administration. All blood samples are diluted with 60 μL of water containing 1.7 mg/mL EDTA and kept at -80 °C until analysis[1]. Rats[1] Twelve male Sprague-Dawley rats are used. All animals are 6-9 weeks old at the time of study and weighed between 200 and 300 g. Animals (n=3 per dosing route) are dosed with 10 or GNE-781 at 1 mg/kg iv (in propyl ethylene glycol 400 (35% v/v) and water (65% v/v)) or 5 mg/kg po (suspended in 0.5% w/v methylcellulose, 0.2% w/v Tween 80). Food and water are available ad libitum to animals in the iv groups. Animals in po groups are fasted overnight and food withheld until 4 h postdose. Approximately 250 μL of blood are collected via the catheter at 0.033, 0.083, 0.25, 0.5, 1, 2, 4, 8, and 24 h after the intravenous or oral administration. All blood samples are collected into tubes containing 5 μL of 0.5 M K2EDTA and processed for plasma. Samples are centrifuged (2500g for 15 min at 4°C) within 1 h of collection, and plasma samples are kept at -80 °C until analysis[1]. In Vivo PK of 10 and 19 (GNE-781)[1] Mouse PK: Twelve female CD-1 mice were used. All animals were 6–9 weeks old at the time of study and weighed between 20 and 35 g. Animals (n = 3 per dosing route) were dosed with 10 or 19 at 1 mg/kg iv (in propyl ethylene glycol 400 (35% v/v) and water (65% v/v)) or 5 mg/kg po (suspended in 0.5% w/v methylcellulose, 0.2% w/v Tween 80). Food and water were available ad libitum to all animals. Serial blood samples (15 μL) were collected by tail nick at 0.033, 0.083, 0.25, 0.5, 1, 3, 8, and 24 h after the intravenous administration and 0.083, 0.25, 0.5, 1, 3, 8, and 24 h after the oral administration. All blood samples were diluted with 60 μL of water containing 1.7 mg/mL EDTA and kept at −80 °C until analysis. Rat PK: [1] Twelve male Sprague–Dawley rats were used. All animals were 6–9 weeks old at the time of study and weighed between 200 and 300 g. Animals (n = 3 per dosing route) were dosed with 10 or 19 at 1 mg/kg iv (in propyl ethylene glycol 400 (35% v/v) and water (65% v/v)) or 5 mg/kg po (suspended in 0.5% w/v methylcellulose, 0.2% w/v Tween 80). Food and water were available ad libitum to animals in the iv groups. Animals in po groups were fasted overnight and food withheld until 4 h postdose. Approximately 250 μL of blood were collected via the catheter at 0.033, 0.083, 0.25, 0.5, 1, 2, 4, 8, and 24 h after the intravenous or oral administration. All blood samples were collected into tubes containing 5 μL of 0.5 M K2EDTA and processed for plasma. Samples were centrifuged (2500g for 15 min at 4 °C) within 1 h of collection, and plasma samples were kept at −80 °C until analysis. View More
Dog PK: [1] |
|
| 参考文献 | ||
| 其他信息 |
Inhibition of the bromodomain of the transcriptional regulator CBP/P300 is an especially interesting new therapeutic approach in oncology. Researchers recently disclosed in vivo chemical tool 1 (GNE-272) for the bromodomain of CBP that was moderately potent and selective over BRD4(1). In pursuit of a more potent and selective CBP inhibitor, we used structure-based design. Constraining the aniline of 1 into a tetrahydroquinoline motif maintained potency and increased selectivity 2-fold. Structure-activity relationship studies coupled with further structure-based design targeting the LPF shelf, BC loop, and KAc regions allowed us to significantly increase potency and selectivity, resulting in the identification of non-CNS penetrant 19 (GNE-781, TR-FRET IC50 = 0.94 nM, BRET IC50 = 6.2 nM; BRD4(1) IC50 = 5100 nΜ) that maintained good in vivo PK properties in multiple species. Compound 19 displays antitumor activity in an AML tumor model and was also shown to decrease Foxp3 transcript levels in a dose dependent manner.[1]
Researchers have identified a highly potent and selective in vivo probe (19,GNE-781) of the CBP bromodomain that is suitable to interrogate the biology of CBP without the complication of BET inhibition. Our studies began with recently disclosed 1 (TR-FRET IC50 = 20 nM, BRET IC50 = 410 nM, BRD4 IC50 = 13,000 nΜ) that was moderately potent for the bromodomain of CBP and 650-fold selective over BRD4. Constraining the aniline of 1 into tetrahydroquinoline 3 maintained potency and increased selectivity by 2-fold over 1. Structure–activity relationship studies coupled with structure-based design targeting the LPF shelf, BC loop, and KAc regions allowed us to identify 10 (TR-FRET IC50 = 1.1 nM, BRET IC50 = 12 nM, BRD4 IC50 = 4200 nΜ). Further profiling of this compound revealed that it penetrated into the CNS, resulting in adverse CNS effects. Subsequent optimization focused on increasing tPSA with the addition of a hydrogen bond donor. This was accomplished with conversion of the Asn-binding acetamide of 10 to a methyl urea, enabling identification of non-CNS penetrant 19 (TR-FRET IC50 = 0.94 nM, BRET IC50 = 6.2 nM, BRD4(1) IC50 = 5100 nΜ) that demonstrated an appropriate balance of cell potency, selectivity (5425-fold over BRD4), and in vivo PK. The exquisite potency and selectivity of 19 enables the clear delineation of pharmacological effects from the inhibition of CBP over the BET bromodomains. In vivo, 19 modulates MYC expression that corresponds with antitumor activity in an AML tumor model. Additional in vitro studies with 19 showed that this compound impaired FOXP3 expression and Treg function, further suggesting CBP bromodomain inhibition as a novel small molecule approach for cancer immunotherapy.[1] |
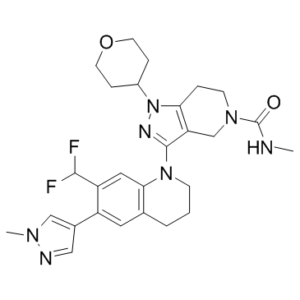
| 分子式 |
C27H33F2N7O2
|
|---|---|
| 分子量 |
525.593432188034
|
| 精确质量 |
525.27
|
| 元素分析 |
C, 61.70; H, 6.33; F, 7.23; N, 18.65; O, 6.09
|
| CAS号 |
1936422-33-1
|
| PubChem CID |
132275066
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| LogP |
2.7
|
| tPSA |
80.4Ų
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
38
|
| 分子复杂度/Complexity |
833
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
CQCWHSDMJBAGDC-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C27H33F2N7O2/c1-30-27(37)34-9-5-23-22(16-34)26(32-36(23)19-6-10-38-11-7-19)35-8-3-4-17-12-20(18-14-31-33(2)15-18)21(25(28)29)13-24(17)35/h12-15,19,25H,3-11,16H2,1-2H3,(H,30,37)
|
| 化学名 |
3-[7-(difluoromethyl)-6-(1-methylpyrazol-4-yl)-3,4-dihydro-2H-quinolin-1-yl]-N-methyl-1-tetrahydropyran-4-yl-6,7-dihydro-4H-pyrazolo[4,3-c]pyridine-5-carboxamide
|
| 别名 |
GNE781; GNE 781; 3-(7-(Difluoromethyl)-6-(1-methyl-1H-pyrazol-4-yl)-3,4-dihydroquinolin-1(2H)-yl)-N-methyl-1-(tetrahydro-2H-pyran-4-yl)-1,4,6,7-tetrahydro-5H-pyrazolo[4,3-c]pyridine-5-carboxamide; CHEMBL4097025; 3-[7-(difluoromethyl)-6-(1-methyl-1H-pyrazol-4-yl)-3,4-dihydroquinolin-1(2H)-yl]-N-methyl-1-(oxan-4-yl)-1,4,6,7-tetrahydro-5H-pyrazolo[4,3-c]pyridine-5-carboxamide; GNE-781.
|
| HS Tariff Code |
2934.99.03.00
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~190.26 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.87 mg/mL (5.46 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.87 mg/mL (5.46 mM) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1.67 mg/mL (3.18 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 1.67 mg/mL (3.18 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100μL 16.7mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: ≥ 1.67 mg/mL (3.18 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 16.7 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9026 mL | 9.5131 mL | 19.0262 mL | |
| 5 mM | 0.3805 mL | 1.9026 mL | 3.8052 mL | |
| 10 mM | 0.1903 mL | 0.9513 mL | 1.9026 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。