| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
DNMT1/DNA methyltransferase
|
|---|---|
| 体外研究 (In Vitro) |
GSK-3484862(0-10 μM,6 或 14 天)可显着减少 DNA 甲基化 [2]。 GSK-3484862(0-10 μM,4 天)会导致 DNMT1 蛋白水平略有降低 [2]。
在这项研究中,研究人员通过用从两个商业来源获得的不同浓度的化合物处理野生型(WT)或Dnmt1/3a/3b三重敲除(TKO)mESC,确定了GSK-3484862的细胞毒性和最佳浓度。10µM或以下的浓度很容易耐受14天的培养。已知的DNA甲基化靶点,如种系基因和GLN家族转座子,在GSK-3484862治疗开始后的2天内上调。相比之下,5-氮杂胞苷和地西他滨诱导了甲基化基因的较弱上调和广泛的细胞死亡。全基因组亚硫酸氢盐测序显示,用GSK-3484862治疗诱导了显著的DNA甲基化损失,在用GSK-344862治疗6天后,Gloval CpG甲基化水平从WT mESC的近70%下降到不到18%。处理过的细胞显示出与Dnmt1缺陷型mESC中观察到的甲基化水平和模式相似的甲基化。 结论:GSK-3484862介导mESC的显著去甲基化,具有最小的非特异性毒性。 [2] |
| 酶活实验 |
确定GSK-3484862毒性[1]
使用J1 WT和DNMT TKO mESC进行测定,以确定GSK-3484862的最佳浓度和毒性。将30000个细胞接种在预先涂有0.1%明胶的24孔板中。第二天,将培养基换成新鲜mESC培养基或含有DMSO(0.1%或1%)的培养基,用于以下浓度的GSK-3484862:2 pM、20 pM、200 pM、2 nM、20 nM、2µM、20µM(0.1%DMSO)和200µM(1%DMSO)。在接下来的六天里,每天刷新培养基,然后评估细胞形态,然后用0.05%胰蛋白酶-EDTA进行细胞分离以进行细胞计数。[1] 接下来,在重复实验中评估了GSK-3484862的去甲基化功效和长期细胞毒性。为了提高溶解度,在DMSO中重新悬浮后,对GSK-3484862进行超声波处理。来自两家公司的GSK-3484862在超声波水浴中以42kHz的频率超声处理6分钟(Sper Scientific)。尽管如此,在培养基中观察到浓度达到或超过20µM的药物沉淀,因此选择了10µM的上限浓度。将WT和DNMT TKO细胞接种在预先涂有0.1%明胶的12孔板中,并从第0天开始在培养基中添加0.1%DMSO、2µM或10µM GSK348862。每天刷新培养基,使用Countess II FL仪器计数细胞,并在接下来的14天内每2-3天传代一次。 |
| 细胞实验 |
细胞活力测定[2]
细胞类型:小鼠胚胎干细胞(mESC、野生型 (WT) 或 Dnmt1/3a/3b 三重敲除 (TKO)) 已测试浓度:2 µM 和 10 µM 孵育时间:6 或 14 天 实验结果:结果 DNA 甲基化急剧丧失6 天后,WT mESC 中的总体 CpG 甲基化水平从近 70% 下降至不到 18%。 蛋白质印迹分析[2] 细胞类型: 小鼠胚胎干细胞(mESC、野生型 (WT) 或 Dnmt1/3a/3b 三重敲除 (TKO)) 测试浓度: 2 µM 和 10 µM 孵育时间: 4 天 实验结果: DNMT1 蛋白水平的产生均适度减少。 |
| 参考文献 |
[1]. Keystone Symposia 2019 - Epigenetics and Human Disease. [2]. The DNMT1 inhibitor GSK-3484862 mediates global demethylation in murine embryonic stem cells. [Epigenetics Chromatin. 2021 Dec 15;14(1):56. https://pubmed.ncbi.nlm.nih.gov/34906184/ ] |
| 其他信息 |
From a research perspective, GSK-3484862 shows a great deal of promise. 5-Azanucleosides have substantial non-specific toxicity and work within a narrow concentration band. We observed reactivation of some methylated genes after treatment with 0.3 µM 5-azacytidine, but very few cells survived at this concentration, while 0.1 µM 5-azacytidine was inadequate to reactivate methylated genes. Furthermore, we had to allow two days for colonies of surviving cells to emerge in culture. By contrast, 2 µM or 10 µM GSK-3484862 reactivated methylated gene expression and produced only modest reduction in growth, most noticeable between days six and 10 of treatment. This reduced growth may reflect specific activity of the compound. When mESCs undergo demethylation mediated by treatment with MEK inhibitor, Glycogen Synthase Kinase 3 inhibitor and high concentration of ascorbic acid, they have a burst of transposon expression and undergo reconfiguration of heterochromatin state approximately during this interval [2].
DNA methylation of the GSK-3484862-treated mESCs never fell below 16% regardless of the dosage used or time of treatment, and methylated genes were not reactivated to the extent observed in the TKO cells. This likely reflects the high level of DNMT3A and DNMT3B activity in mESCs, as evidenced by the similar level of DNA methylation in published Dnmt1 KO mESCs. The Dnmt1 deficient or inhibited cells potentially reach an equilibrium in which methylation is constantly added by DNMT3A and DNMT3B and lost through replication and Tet-protein activity. Other cell types may respond to Dnmt1 inhibition differently. Most somatic and cancer cells do not express such high levels of the de novo DNA methyltransferases and may not be able to maintain such high levels of DNA methylation in the absence of Dnmt1 activity. At the same time, somatic or cancer cells may not survive dramatic DNA methylation loss. Dnmt1 deficient and Dnmt1/3a/3b TKO mESCs are not viable upon differentiation, and DNMT1 becomes essential after uterine implantation. This shift may reflect the fact that mESCs depend heavily on TRIM28 to silence transposons, but upon differentiation of mESCs or uterine implantation of embryos, DNA methylation gains importance for transposon repression. Thus, researchers working with other cell types may well observe specific toxicity at lower doses, and indeed GSK-3484862 and related compounds show a striking effect in leukemias. We also cannot rule out that non-specific toxicity may occur in some cell types, a result suggested by the GSK-3484862’s ability to halt mouse development at relatively low concentrations. Nonetheless, this novel DNA methyltransferase inhibitor appears to be a substantial improvement over 5-azanucleosides and a promising research tool. Conclusions GSK-3484862 mediates dramatic demethylation in murine embryonic stem cells. With regard to both activation of methylated genes and non-specific toxicity, GSK-3484862 performs far better than 5-azanucleosides. |
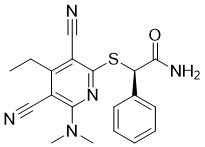
| 分子式 |
C19H19N5OS
|
|---|---|
| 分子量 |
365.452061891556
|
| 精确质量 |
365.131
|
| 元素分析 |
C, 62.45; H, 5.24; N, 19.16; O, 4.38; S, 8.77
|
| CAS号 |
2170136-65-7
|
| 相关CAS号 |
(Rac)-GSK-3484862;2170136-02-2
|
| PubChem CID |
132233666
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| LogP |
3
|
| tPSA |
132
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
585
|
| 定义原子立体中心数目 |
1
|
| SMILES |
S(C1=C(C#N)C(=C(C#N)C(=N1)N(C)C)CC)[C@@H](C(N)=O)C1C=CC=CC=1
|
| InChi Key |
KIEQQZZDWUNUQK-MRXNPFEDSA-N
|
| InChi Code |
InChI=1S/C19H19N5OS/c1-4-13-14(10-20)18(24(2)3)23-19(15(13)11-21)26-16(17(22)25)12-8-6-5-7-9-12/h5-9,16H,4H2,1-3H3,(H2,22,25)/t16-/m1/s1
|
| 化学名 |
(R)-2-((3,5-dicyano-6-(dimethylamino)-4-ethylpyridin-2-yl)thio)-2-phenylacetamide
|
| 别名 |
GSK3484862; GSK-3484862; 2170136-65-7; GSKMI-714; (2R)-2-[3,5-dicyano-6-(dimethylamino)-4-ethylpyridin-2-yl]sulfanyl-2-phenylacetamide; SCHEMBL19717424; BDBM491120; US10975056, Example 64; NSC825088; GSK 3484862
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~20.83 mg/mL (~57.00 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.08 mg/mL (5.69 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.08 mg/mL (5.69 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7364 mL | 13.6818 mL | 27.3635 mL | |
| 5 mM | 0.5473 mL | 2.7364 mL | 5.4727 mL | |
| 10 mM | 0.2736 mL | 1.3682 mL | 2.7364 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。