| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 靶点 |
RIPK3
GSK-843 (3-10 μM; 18 h) induces apoptosis[1]. GSK-843 (0.3-3 μM; 18 h) inhibits virus- and TNF-induced cell necrosis[2]. Researchers previously identified the RIP3i GSK’843 and GSK’872 by screening conventional small-molecule libraries (Kaiser et al., 2013a) (Figure 1A). These compounds bound RIP3 kinase domain with high affinity (IC50 = 8.6 nM and 1.8 nM, respectively; Figure 1B) and inhibited kinase activity (IC50 = 6.5 nM and 1.3 nM, respectively; Figure 1C). When assayed individually at 1 μM, the three structurally distinct compounds failed to inhibit most of 300 human protein kinases tested, with GSK’840 showing the best profile (Figure S1B; Table S1). All compounds failed to inhibit RIP1 kinase when tested directly (data not shown). Taken together, this demonstrates that GSK’840, GSK’843, and GSK’872 bind to RIP3 kinase domain and inhibit enzyme activity with minimal cross-reactivity[1]. |
|---|---|
| 体外研究 (In Vitro) |
GSK-843(3-10 μM;18 小时)诱导细胞凋亡[1]。 GSK-843(0.3-3 μM;18 小时)抑制病毒和 TNF 诱导的细胞坏死[2]。
研究人员之前通过筛选常规小分子文库确定了RIP3i GSK ' 843和GSK ' 872 (Kaiser et al., 2013a)(图1A)。这些化合物以高亲和力结合RIP3激酶结构域(IC50分别为8.6 nM和1.8 nM);图1B)和抑制的激酶活性(IC50分别为6.5 nM和1.3 nM;图1 c)。当在1 μM下单独检测时,这三种结构不同的化合物对300种人蛋白激酶的大部分都没有抑制作用,其中GSK 840表现出最好的抑制效果(图S1B;表S1)。当直接测试时,所有化合物都不能抑制RIP1激酶(数据未显示)。综上所述,这表明GSK ' 840、GSK ' 843和GSK ' 872结合到RIP3激酶结构域,并以最小的交叉反应性抑制酶活性[1]。 GSK'843 以浓度依赖性方式抑制人 HT-29 细胞中 TNF 诱导的坏死性凋亡,其 IC50 比无细胞生化实验高 100 至 1000 倍 [1]。 GSK'843 在 0.04 至 1 μM 的浓度范围内,可抑制小鼠细胞中的坏死性凋亡,这些细胞包括骨髓来源巨噬细胞、硫代乙醇酸盐诱导的腹膜巨噬细胞和 3T3SA 成纤维细胞 [1]。 GSK'843 可抑制由 poly(I:C) 在泛 caspase 抑制剂 zVAD 存在下触发的 Toll 样受体 3 诱导的坏死性凋亡 [1]。 在高浓度(3 μM 和 10 μM)下,GSK'843 会在多种细胞类型中触发 caspase 依赖性凋亡性细胞死亡,该死亡可被 zVAD 逆转 [1]。 这种凋亡的特征包括 caspase-3 被切割、效应 caspase 活性增加、膜起泡以及电镜下的凋亡形态 [1]。 GSK'843 的促凋亡活性需要 RIP3 的存在,这在 RIP3 低表达的 NIH3T3 细胞敏感性降低、Rip3/- MEF 细胞抵抗以及用人类 RIP3 重建的 Rip3/- MEF 恢复敏感性等实验中得到证实 [1]。 GSK'843 诱导的凋亡不依赖于促坏死机器,但依赖于 RHIM 信号传导,因为 RHIM 突变的人类 RIP3 无法赋予敏感性 [1]。 用 GSK'843 处理会以浓度依赖性方式驱动 RIP1-FADD-cFLIPL-Casp8 复合物的组装,尤其是在 zVAD 存在下该复合物更稳定 [1]。 这种凋亡需要 RIP1、FADD、cFLIPL 和 Casp8,但不需要 RIP1 激酶活性,因为来自 Rip1K45A/K45A 激酶失活敲入小鼠的细胞仍然敏感 [1]。 来自 MCMV 的病毒 RIP 激活抑制剂(vIRA)可抑制 GSK'843 诱导的凋亡 [1]。 GSK'843 显示出最小的交叉反应性,在 1 μM 浓度下对测试的 300 种人类蛋白激酶中的大多数没有抑制作用,并且不直接抑制 RIP1 激酶 [1]。 |
| 体内研究 (In Vivo) |
Rip3K51A/K51A激酶死亡敲入小鼠存活[1]
RIP3激酶死亡突变体的行为支持在D161N突变敲入小鼠中观察到的惊人的妊娠中期致命性(Newton等人,2014),并预测无毒突变体将出现相反的结果。当产生Rip3K51A/K51A激酶死亡敲入小鼠时,小鼠明显具有活力和生育能力(图7A和7B)。该突变株对妊娠中期或围产期死亡没有表现出任何易感性。为了确定存活的Rip3K51A/K51A突变体是否像致死性Rip3D161N/D161N突变体(Newton et al., 2014)一样,挽救了Casp8−/−胚胎的胚胎致死性,我们进行了杂交,并以预期的孟德尔频率挽救了存活和可育的Casp8−/−Rip3K51A/K51A小鼠(图7B和S6A)。这扩展了先前对Casp8−/−Rip3−/−小鼠的拯救(Kaiser et al., 2011;Oberst et al., 2011;Zhang et al., 2011)清楚地表明,在没有Rip3D161N/D161N突变并发症的casp8缺陷胚胎妊娠中期死亡中,早衰RIP3酶活性的贡献(Newton et al., 2014)。 本研究描述了 Rip3K51A/K51A 激酶失活敲入小鼠的构建和表征,这些小鼠存活且可育,旨在证明 RIP3 激酶活性对生命并非必需,并为无毒激酶抑制剂的效果提供模型。[1] |
| 酶活实验 |
RIP3高通量筛选荧光极化(FP)试验用于筛选与RIP3激酶结构域荧光标记探针(GSK ' 657)结合竞争的小分子化合物文库(Pope et al., 1999)。文库化合物抑制RIP3激酶活性的能力是通过使用ADP-Glo测量ATP消耗的实验来评估的(Li et al., 2009)。编码库技术筛选按照前面描述进行(Deng et al., 2012)。kinome面板的体外分析由Reaction Biology Corporation使用“HotSpot”分析平台进行(Anastassiadis等,2011)。Kinome树表示是使用Kinome Mapper生成的。[1]
通过结合和激酶抑制实验,以纯化的、杆状病毒表达的重组人 RIP3 激酶结构域(氨基酸 2-328)为靶标,筛选小分子抑制剂候选物 [1]。 使用荧光偏振法测定化合物与 RIP3 激酶结构域的结合亲和力 [1]。 使用 ADP-Glo 法测定化合物对重组 RIP3 激酶活性的抑制效力 [1]。 通过在 1 μM 浓度下测试化合物对 300 种人类蛋白激酶组的选择性进行 profiling [1]。 |
| 细胞实验 |
细胞活力、caspase活性和显微观察[1]
使用Cell Titer-Glo发光细胞活力测定试剂盒间接检测ATP,使用细胞毒性LDH测定试剂盒检测乳酸脱氢酶释放,使用IncuCyte对SYTOX Green的摄取。利用caspase - glo 3/7活性测定系统和caspase - glo 8活性测定系统分别测定效应物caspase活性。如前所述(Tandon和Mocarski, 2008)进行透射电子显微镜(TEM),并在Emory electron microscopy Core使用JEOL JEM-1400透射电子显微镜获得图像。 细胞活力测定[1] 将L929细胞(5000细胞/孔)、BMDM细胞(30000细胞/孔)、NIH3T3细胞(10000细胞/孔)、3T3-SA细胞(10000细胞/孔)、SVEC4-10细胞(10000细胞/孔)分别接种到康宁96孔组织培养板(3610)中。在大多数实验中,根据制造商的说明,使用cell Titer-Glo发光细胞活力测定试剂盒通过测量细胞内ATP水平来评估细胞活力,并将结果与对照培养物相比较。 坏死性凋亡抑制实验:用刺激剂处理人 HT-29 细胞或小鼠细胞,并在不同浓度的 GSK'843 存在下培养。处理 18-24 小时后,通常通过测量细胞 ATP 水平来评估细胞活力 [1]。 凋亡诱导实验:用高浓度 GSK'843 处理各种细胞系,并在存在或不存在 caspase 抑制剂的情况下培养。通过活力测定、显微镜检查膜通透性、流式细胞术检测 caspase-3 切割或测量 caspase-3/7 和 caspase-8 酶活性来监测细胞死亡 [1]。 复合物形成的生化分析:在用 GSK'843 处理后,对细胞裂解物中的 FADD 或 FLAG 标记的 RIP3 进行免疫沉淀,然后对 RIP3、RIP1、FADD、Casp8 和 cFLIP 等组分进行免疫印迹分析 [1]。 基因需求验证:使用基因敲除细胞、激酶失活突变细胞、siRNA/shRNA 介导的敲低以及重建实验来验证特定基因在药物作用中的必要性 [1]。 病毒抑制剂作用测试:在用 GSK'843 处理前,用表达 MCMV vIRA 或其突变体的逆转录病毒转导细胞 [1]。 |
| 动物实验 |
Mice, infections, and organ Harvests [1]
RIP3K51A/K51A mice and RIP1K45A/K45A (Berger et al., 2014) were generated at Genoway (Lyon, France). Rip3/ (Newton et al., 2004), Tnf/ (Pasparakis et al., 1997), Rip3/ Casp8/ (Kaiser et al., 2011), Rip1/ Rip3/ Casp8/ , and Rip1/ Rip3+/ Casp8/ (Kaiser et al., 2014) mice have been described. C57BL/6 mice were from Jackson Laboratory and Rip3−/− mice Ripk3tm1Vmd) were from Genentech (Newton et al., 2004). WT MCMV strain K181, as well as M45mutRHIM and lacZ-expressing RM461 have been described previously (Stoddart et al., 1994; Upton et al., 2010). Mice were injected intraperitoneally with 106 PFU MCMV M45mutRHIM. 14 days post infection mice were re-injected intraperitoneally with MCMV lacZ expressing strain RM427 and organs harvested 4 days later. Organ titers were performed as previously described (Upton et al., 2010). Generation of a Rip3K51A/K51A kinase inactive knockin mice [1] The knockin strategy was designed and performed by genOway. The Rip3 gene-targeting vector was constructed from genomic C57BL/6 mouse strain DNA. The K51A point mutation was inserted into Rip3 exon 2 while a neomycin resistance gene cassette was inserted in intron 3 (flanked by FRT sites for further Flp-mediated excision). Exon 2 Including the K51A point mutation was flanked by loxP sites enabling access to constitutive or conditional deletion using Cre-mediated recombination. This manuscript does not describe specific animal experiments involving the administration of the compound GSK'843. The in vivo conclusions are based on the phenotypic study of genetically engineered Rip3K51A/K51A knockin mice [1]. The generation of Rip3K51A/K51A mice involved standard gene targeting techniques in embryonic stem cells, followed by breeding to obtain homozygous mutants. Mice were used for viability/fertility assessment, cell isolation (MEFs, BMDMs), and infection models with MCMV [1]. For the MCMV infection model, age-matched mice of different genotypes (WT, Rip3/- , Rip3K51A/K51A) were infected intraperitoneally with MCMV-M45mutRHIM virus. Viral titers in spleen and liver were determined by plaque assay 3 days post-infection [1]. For immune response analysis, mice were primed with MCMV-M45mutRHIM and later challenged with a lacZ-expressing MCMV. Splenocytes were harvested 4 days post-challenge and stimulated with M45-specific peptide to measure IFNγ and TNFα production in CD8+ T cells by intracellular cytokine staining [1]. |
| 毒性/毒理 (Toxicokinetics/TK) |
The primary toxicity noted for GSK'843 in this study is its concentration-dependent induction of caspase-8-dependent apoptosis in cell culture at high concentrations (≥3 μM), which is an on-target effect related to RIP3 conformational change and RHIM-signaling activation [1].
|
| 参考文献 | |
| 其他信息 |
Receptor-interacting protein kinase 3 (RIP3 or RIPK3) has emerged as a central player in necroptosis and a potential target to control inflammatory disease. Here, three selective small-molecule compounds are shown to inhibit RIP3 kinase-dependent necroptosis, although their therapeutic value is undermined by a surprising, concentration-dependent induction of apoptosis. These compounds interact with RIP3 to activate caspase 8 (Casp8) via RHIM-driven recruitment of RIP1 (RIPK1) to assemble a Casp8-FADD-cFLIP complex completely independent of pronecrotic kinase activities and MLKL. RIP3 kinase-dead D161N mutant induces spontaneous apoptosis independent of compound, whereas D161G, D143N, and K51A mutants, like wild-type, only trigger apoptosis when compound is present. Accordingly, RIP3-K51A mutant mice (Rip3(K51A/K51A)) are viable and fertile, in stark contrast to the perinatal lethality of Rip3(D161N/D161N) mice. RIP3 therefore holds both necroptosis and apoptosis in balance through a Ripoptosome-like platform. This work highlights a common mechanism unveiling RHIM-driven apoptosis by therapeutic or genetic perturbation of RIP3.[1]
Toll-like receptor (TLR) signaling is triggered by pathogen-associated molecular patterns that mediate well established cytokine-driven pathways, activating NF-κB together with IRF3/IRF7. In addition, TLR3 drives caspase 8-regulated programmed cell death pathways reminiscent of TNF family death receptor signaling. We find that inhibition or elimination of caspase 8 during stimulation of TLR2, TLR3, TLR4, TLR5, or TLR9 results in receptor interacting protein (RIP) 3 kinase-dependent programmed necrosis that occurs through either TIR domain-containing adapter-inducing interferon-β (TRIF) or MyD88 signal transduction. TLR3 or TLR4 directly activates programmed necrosis through a RIP homotypic interaction motif-dependent association of TRIF with RIP3 kinase (also called RIPK3). In fibroblasts, this pathway proceeds independent of RIP1 or its kinase activity, but it remains dependent on mixed lineage kinase domain-like protein (MLKL) downstream of RIP3 kinase. Here, we describe two small molecule RIP3 kinase inhibitors and employ them to demonstrate the common requirement for RIP3 kinase in programmed necrosis induced by RIP1-RIP3, DAI-RIP3, and TRIF-RIP3 complexes. Cell fate decisions following TLR signaling parallel death receptor signaling and rely on caspase 8 to suppress RIP3-dependent programmed necrosis whether initiated directly by a TRIF-RIP3-MLKL pathway or indirectly via TNF activation and the RIP1-RIP3-MLKL necroptosis pathway.[2] GSK'843 is a selective small-molecule inhibitor of the RIP3 kinase, identified alongside GSK'872 from conventional small molecule library screening [1]. The study reveals a dual role of RIP3. While its kinase activity drives MLKL-dependent necroptosis, the RIP3 protein itself can scaffold a pro-apoptotic complex. High concentrations of GSK'843 (and other RIP3 inhibitors) induce a conformational change in RIP3, promoting RHIM-dependent oligomerization and recruitment of RIP1, FADD, cFLIPL, and Casp8, leading to apoptosis independent of kinase activity [1]. This apoptotic activity poses a potential challenge for developing RIP3 kinase inhibitors as anti-inflammatory therapeutics, as it may represent an undesirable on-target toxicity [1]. The study differentiates between various RIP3 kinase-dead mutants. While the D161N mutation spontaneously induces apoptosis (mimicking high-dose inhibitor), the K51A mutation does not, unless the inhibitor is present. This indicates that kinase activity loss itself is not toxic, but specific perturbations (certain mutations or inhibitor binding at high concentration) can convert RIP3 into a pro-apoptotic adapter [1]. The viability of Rip3K51A/K51A mice serves as a proof-of-concept that eliminating RIP3 kinase activity in vivo is not inherently lethal and does not compromise immunocompetence, suggesting that non-toxic RIP3 inhibitors are feasible if they avoid triggering the pro-apoptotic conformational change [1]. |
| 分子式 |
C₁₉H₁₅N₅S₂
|
|---|---|
| 分子量 |
377.49
|
| 精确质量 |
377.076
|
| 元素分析 |
C, 60.46; H, 4.01; N, 18.55; S, 16.99
|
| CAS号 |
1601496-05-2
|
| 相关CAS号 |
1601496-05-2
|
| PubChem CID |
91885439
|
| 外观&性状 |
Light yellow to yellow solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
640.0±55.0 °C at 760 mmHg
|
| 闪点 |
340.8±31.5 °C
|
| 蒸汽压 |
0.0±1.9 mmHg at 25°C
|
| 折射率 |
1.832
|
| LogP |
4.9
|
| tPSA |
126
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
522
|
| 定义原子立体中心数目 |
0
|
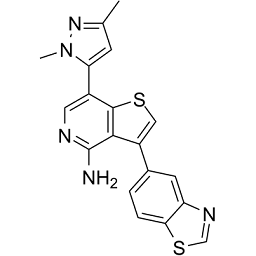
| SMILES |
S1C=C(C2C=CC3=C(C=2)N=CS3)C2C(N)=NC=C(C3=CC(C)=NN3C)C1=2
|
| InChi Key |
BPKSNNJTKPIZKR-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H15N5S2/c1-10-5-15(24(2)23-10)12-7-21-19(20)17-13(8-25-18(12)17)11-3-4-16-14(6-11)22-9-26-16/h3-9H,1-2H3,(H2,20,21)
|
| 化学名 |
3-(1,3-benzothiazol-5-yl)-7-(2,5-dimethylpyrazol-3-yl)thieno[3,2-c]pyridin-4-amine
|
| 别名 |
GSK843; GSK-843; GSK 843; 1601496-05-2; GSK-843; GSK843; GSK'843; 3-(1,3-benzothiazol-5-yl)-7-(1,3-dimethyl-1H-pyrazol-5-yl)thieno[3,2-c]pyridin-4-amine; CHEMBL4441118; 3-(1,3-BENZOTHIAZOL-5-YL)-7-(2,5-DIMETHYLPYRAZOL-3-YL)THIENO[3,2-C]PYRIDIN-4-AMINE; 3-(benzo[d]thiazol-5-yl)-7-(1,3-dimethyl-1H-pyrazol-5-yl)thieno[3,2-c] pyridin-4-amine; . GSK'843
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~50 mg/mL (~132.5 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 4.8 mg/mL (12.72 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 48.0mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.51 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6491 mL | 13.2454 mL | 26.4908 mL | |
| 5 mM | 0.5298 mL | 2.6491 mL | 5.2982 mL | |
| 10 mM | 0.2649 mL | 1.3245 mL | 2.6491 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|