| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| 1g | |||
| Other Sizes |
| 靶点 |
TRPV4/transient receptor potential vanilloid 4
|
|---|---|
| 体外研究 (In Vitro) |
GSK1016790A (0.1-1000 nM) 当添加到表达人 TRPV4 的小鼠和人胚胎肾 (HEK) 细胞中时,会引起 Ca2+ 流入(EC50 值为 18 和 2.1 nM),并且在剂量为 1 nM 时,TRPV4 全细胞电流。在部分神经元中,(100 nM) 处理会导致细胞内 Ca2+ 快速升高 [2]。
TRP超家族的瞬时受体电位(TRP)香草醛4(TRPV4)成员最近与许多生理过程有关。在这项研究中,我们描述了一种小分子TRPV4通道激活剂(N-(1S)-1-{[4-(2S)-2-{[(2,4-二氯苯基)磺酰基]氨基}-3-羟基丙酰基)-1-哌嗪基]羰基}-3-甲基丁基)-1-苯并噻吩-2-甲酰胺(GSK1016790A),我们将其用作研究TRPV4在膀胱中作用的有价值的工具。GSK1016790A在小鼠和表达人TRPV4的人胚胎肾(HEK)细胞中引发Ca2+内流(EC50值分别为18和2.1 nM),并且在浓度高于1 nM时,它引发TRPV4全细胞电流的剂量依赖性激活。相比之下,TRPV4激活剂4α佛波醇12,13-癸酸酯(4αPDD)在激活TRPV4电流方面的效力比GSK1016790A低300倍。在TRPV4+/+小鼠膀胱平滑肌(UBSM)和尿道上皮中检测到TRPV4 mRNA。Western印迹和免疫组织化学显示,UBSM和尿路上皮中都有蛋白质表达,而TRPV4-/-膀胱中则没有。在体外,无论是否存在尿路上皮,GSK1016790A激活TRPV4都会收缩TRPV4+/+小鼠膀胱,这种作用在TRPV4-/-膀胱中未被检测到。与对TRPV4-HEK全细胞电流的影响一致,与GSK1016790A相比,4αPDD显示出较弱的收缩膀胱条的能力[1]。 位于肌间神经元上的TRPV4介导GSK1016790A的作用[2] 然后对从WT和TRPV4-/-小鼠的近端和远端结肠分离和培养的肌间神经元以及表达TRPV4的HEK-293细胞进行研究,以进一步证实GSK1016790A选择性激活TRPV4通道,并阐明细胞外和细胞内Ca2+在GSK101679A诱导的效应中的作用。 GSK1016790A(100 nM)处理引起神经元亚群细胞内Ca2+快速升高(48± 2 % 或1143/2289个神经元 = 12) (图3a)。反应主要在刺激期间持续。非神经元细胞也对TRPV4激活剂有反应。这些是具有多个突起的小细胞,表明在这些培养条件下,Cajal间质细胞或肠神经胶质细胞能够表达TRPV4。然而,由于本研究的重点是神经元TRPV4的表达,因此这些细胞的特性没有得到进一步的表征。 在用TRPV4选择性拮抗剂HC067047(10μM,预处理30分钟)预处理后,对GSK1016790A(100 nM)的反应被有效消除。HC处理后,神经元对卡巴胆碱(10μM)和KCl(50 mM)的反应得以保留,表明神经元存活率没有受到显著影响(图3b)。在用非选择性离子通道抑制剂RuR(1μM,预处理30分钟)预处理后,对GSK1016790A的反应也出现了类似的阻断。通过比较野生型和TRPV4-/-小鼠,证实了肌间神经元对TRPV4的功能表达(图3b-d)。GSK1016790A未能提高TRPV4-/-小鼠培养的神经元的细胞内Ca2+水平。对CCh和KCl的反应不受影响(图3d)。 在稳定表达人TRPV4的HEK细胞中检测了GSK1016790A的特异性。GSK1016790A(10−10–10−6 M)以浓度依赖的方式升高细胞内Ca2+(补充信息,图S2),这种作用被RN 1734阻断(5× 10−6 M) 或在未诱导TRPV4表达的细胞中不存在。我们还观察到GSK1016790A在含有EDTA的无Ca2+缓冲液中没有刺激作用,这表明这种作用需要细胞外钙。 小鼠结肠离体平滑肌条中细胞内钙储存的耗竭(详见补充信息)消除了GSK1016790A诱导的小鼠结肠平滑肌舒张作用(补充信息,图S3)。因此,我们可以假设细胞内钙离子的存在是GSK1016790A诱导效应所必需的。 |
| 体内研究 (In Vivo) |
在小鼠中,GSK1016790A(0.001-0.1 mg/kg;腹腔注射)以剂量依赖性方式抑制转录转运时间 [2]。 GSK1016790A(0.1-1000 nM;假设 10 分钟)以浓度抑制方式产生显着抑制作用 [2]。
在体内,TRPV4+/+和TRPV4-/-小鼠的尿动力学显示TRPV4--/-小鼠的膀胱容量增强。将GSK1016790A输注到TRPV4+/+小鼠的膀胱中可诱导膀胱过度活动,但对TRPV4-/-小鼠没有影响。总体而言,TRPV4在膀胱功能中起着重要作用,包括由于UBSM中TRPV4的表达而收缩膀胱的能力[1]。 GSK1016790A抑制小鼠结肠平滑肌收缩性[2] 接下来,我们使用器官浴在体外表征了TRPV4激活对小鼠回肠和结肠段平滑肌收缩性的影响。GSK1016790A以浓度依赖的方式显著抑制了EFS诱导的离体小鼠结肠条的抽搐收缩(图2a)。RN 1734(10-5M)和RuR(10-6M,图2a)阻断了GSK1016790A在小鼠结肠中的作用。为了证实GSK1016790A的作用是由TRPV4通道介导的,在选择性TRPV1拮抗剂4′-氯-3-甲氧基肉桂酰苯胺(SB 366791)存在下重复实验。SB 366791(10-6M)不影响GSK101679A对小鼠结肠平滑肌收缩性的抑制作用(补充信息,图S1)。所用浓度的GSK1016790A对基础肌张力、静息期活动或用白三醇预收缩的平滑肌没有影响(10−7–2× 10−5 M、 数据未显示; n = 8),不太可能对平滑肌产生直接影响。 GSK1016790A抑制胃肠运动[2] 为了确定TRPV4激活剂对肠道的体外作用是否转化为整个动物,在小鼠胃肠道运动的标准化测试中研究了GSK1016790A的作用。GSK1016790A(0.001–0.1 mg/kg,i.p.)对小鼠的整个肠道转运时间产生剂量依赖性抑制作用,RN 1734(1 mg/kg,i.p..;图4a)逆转了这一作用。GSK1016790A(0.1 mg/kg,i.p.)对TRPV4-/-小鼠没有抑制作用。此外,GSK1016790A对小鼠腹腔注射后的结肠排出产生了剂量依赖性(图4b)和时间依赖性(见图4c)的抑制作用,这被RN 1734(1 mg/kg,腹腔注射)阻断。我们没有观察到GSK1016790A对TRPV4-/-动物结肠珠排出的影响(图4d)。 腹腔注射GSK1016790A(0.1 mg/kg)对小鼠上消化道转运时间没有任何影响(补充信息,图S4)。 因此,本研究使用了两种模拟人类病理生理条件的动物模型,一种可逆的乙酰胆碱酯酶抑制剂新斯的明和应激诱导的高运动。以剂量依赖方式给药GSK1016790A(0.001–0.1 mg/kg,i.p.)可降低新斯的明(2.5μg/kg,i.p..)在小鼠体内诱导的胃肠道高动力(图4e)。此外,腹腔注射GSK1016790A(0.1mg/kg,腹腔注射)减少了暴露于轻度应激的小鼠的粪便颗粒产量(图4f)。GSK1016790A在该模型中对TRPV4-/-小鼠没有影响(图4f)。 NOS通过可溶性鸟苷酸环化酶作为下游效应器参与TRPV4诱导的小鼠结肠中GSK1016790A的作用[2] NO是一种主要的抑制性神经递质,由抑制性运动神经元和下行中间神经元产生。在这里,我们研究了NO在TRPV4激动剂对结肠收缩的抑制作用中的作用,并使用已建立的NO成像技术进一步确定了TRPV4下游的效应器。 实验表明,GSK1016790A(10-7M)刺激了小鼠结肠LMMP制剂中NO的释放(图5a,b)。GSK1016790A的作用被RuR(10-6M)、SMTC(10-5M)和1400W(10-5Ms;图5b,c)联合阻断。由于NO是一种易于扩散穿过细胞膜的气态神经递质,因此确定肌间神经丛内NO的细胞来源是一个重大挑战,因为所有细胞都负载有对NO敏感的染料。细胞外NO清除剂2-苯基-4,4,5,5-四甲基咪唑啉-1-氧基-3-氧化物(PTIO,10-4M)的应用阻止了NO的细胞外运动,从而允许基于细胞荧光定位产生NO的细胞(相对于对NO的反应)。肌间神经丛内响应GSK1016790A产生NO的神经元百分比为40 ± 6 (n = 3). 当SMTC和PTIO联合应用时,神经元对GSK1016790A的反应被完全抑制,而在1400W和PTIO存在的情况下,神经元对GSK1016790A的反应与单独使用GSK相比没有变化(图5b)。 |
| 酶活实验 |
离体平滑肌条的体外实验[2]
按照所述检查孤立肠段的收缩性(补充信息)GSK1016790A(N-((1S)-1-{[4-(2S)-2-{[(2,4-二氯苯基)磺酰基]氨基}-3-羟基丙酰基)-1-哌嗪基]羰基}-3-甲基丁基)-1-苯并噻吩-2-甲酰胺,10−10–10−6 M)被累积添加到器官浴中,并记录了对电场刺激(EFS;8 Hz)诱导的收缩或舒张的影响。允许每种浓度孵育10分钟。在添加GSK1016790A之前,使用四次连续抽搐收缩或放松的平均振幅来产生内部对照。收缩或舒张振幅的变化以内部控制的百分比报告。在对照实验中测试了载体(DMSO)的效果。[2] 在单独的实验中,将TRPV4拮抗剂2,4-二氯-N-异丙基-N-(2-异丙基氨基乙基)苯磺酰胺(RN 1734,10-5M)、TRPV拮抗剂钌红(RuR,10-6M)、NOS-1阻断剂S-甲基-L-硫代瓜氨酸添加GSK1016790A前至少一分钟。拮抗剂和对照实验的效果作为配对试验进行,同时使用八个器官浴。 |
| 细胞实验 |
肌间神经元[Ca2+]i的测定[1]
为了测量单个神经元中的细胞内钙[Ca2+]i),细胞在含有20 mM HEPES和0.1%BSA的Hank's平衡盐溶液(HBSS)中装载Fura2 AM酯(2μM,30分钟,37°C)。在37°C下使用Leica DMI-6000B成像系统和×10干物镜测量荧光。在340和380nm激发波长和510nm发射波长下收集图像(间隔5-s)。使用ImageJ软件和McMaster生物光子学设施插件(v1.46b;http://imagej.nih.gov/ij). 结果以340/380nm荧光发射比表示,作为细胞内钙([Ca2+]i)的间接测量。依次用GSK1016790A(100 nM,TRPV4激动剂)、卡巴胆碱(1μM,毒蕈碱受体激动剂)和KCl(50 mM)攻击神经元。在某些实验中,细胞在加入激动剂前30分钟用HC067047(10μM,TRPV4拮抗剂)或钌红(10μM)预处理。对GSK1016790A和KCl有反应的细胞,其峰值340/380nm比值大于基线0.1单位,被计为TRPV4阳性神经元。 离体平滑肌条的体外实验[2] 按照所述检查孤立肠段的收缩性(补充信息)。将GSK1016790A(N-((1S)-1-{[4-(2S)-2-{[(2,4-二氯苯基)磺酰基]氨基}-3-羟基丙酰基)-1-哌嗪基]羰基}-3-甲基丁基)-1-苯并噻吩-2-甲酰胺,10−10–10−6 M)累积添加到器官浴中,并记录对电场刺激(EFS;8 Hz)诱导的收缩或舒张的影响。允许每种浓度孵育10分钟。在添加GSK1016790A之前,使用四次连续抽搐收缩或放松的平均振幅来产生内部对照。收缩或舒张振幅的变化以内部控制的百分比报告。在对照实验中测试了载体(DMSO)的效果。 在单独的实验中,在添加前10分钟,将TRPV4拮抗剂2,4-二氯-N-异丙基-N-(2-异丙基氨基乙基)苯磺酰胺(RN 1734,10-5M)、TRPV拮抗剂钌红(RuR,10-6M)、NOS-1阻断剂S-甲基-L-硫代瓜氨酸(SMTC,10-5MA)、NOS-2阻断剂N-(3-氨基甲基)苄基乙脒(1400W,10-6MA)和可溶性鸟苷酸环化酶(sGC)抑制剂1H-[1,2,4]恶二唑并[4,3-a]喹喔啉-1-酮(ODQ)加入器官浴中。GSK1016790A。拮抗剂和对照实验的效果作为配对试验进行,同时使用八个器官浴。 |
| 动物实验 |
Animal/Disease Models: S100β-GFP and TRPV4 knockout (TRPV4-/-) mice [2]
Doses: 0.001, 0.01, 0.1 mg/kg Route of Administration: IP; Single dose Experimental Results: Effect on whole intestinal transit time in mice Dose-dependent inhibition. Produces dose-dependent and time-dependent inhibition of colonic evacuation. Generation of TRPV4−/− Mice. [1] Genomic fragments homologous to the TRPV4 locus were cloned by polymerase chain reaction (PCR) from the E14.1 embryonic stem (ES) cell line using the Expand long template PCR kit (Roche, Palo Alto, CA). Primers for the 5′ homology arm were VR4_5F (5′-TTC TTG TTG ACC CAC AAG AAG CGC CT-3′) and VR4_5R (5′-ATG GTG TCG TTG CGC CCG TTG CTT AGG TT-3′) spanning coding exons 3 to 4; for the 3′ arm, VR4_3F (5′-TTC TTC CAG CCC AAG GAT GAG GGA GGC T-3′) and VR4_3R (5′-AGA TGC CGG GTG TCC TCA TCT GTC ACC... In vivo whole gastrointestinal transit [2] To evaluate the peristaltic motility in the mouse whole intestine, whole gut transit time was measured using the established protocol. Briefly, 0.15 ml of viscous liquid consisting of 5 % Evans blue (a non-absorbable colored marker) and 5 % gum arabic was administered intragastrically to mice, using an 18-gauge animal feeding tube. Fifteen minutes before marker administration, mice were treated (i.p.) with GSK1016790A or vehicle. In selected experiments, the TRPV4 antagonist RN 1734 was injected i.p. 15 min before agonist administration. Immediately after the administration of the marker, mice were returned to individual cages, which were placed on a white sheet in order to facilitate recognition of colored boluses. Time elapsed between intragastric administration of the marker and the excretion of the first colored fecal bolus was considered as time of whole gut transit. In vivo colonic expulsion test [2] Distal colonic expulsion was measured using the following protocol: after an overnight fasting period, GSK1016790A or vehicle was injected i.p. and a pre-warmed (37 °C) glass bead (2 mm) was inserted 2 cm into the distal colon using a silicone pusher. After the bead insertion, mice were placed in individual cages and the time to bead expulsion was determined. Mice that did not expel the bead within 30 min were sacrificed to confirm the presence of the bead in the lumen of the intestine. In vivo fecal pellet output [2] To test the effect of GSK1016790A on stress-induced defecation, non-fasted animals were injected i.p. with GSK1016790A (0.001–0.1 mg/kg) or vehicle 15 min prior to the start of the experiment. Mice were then placed on a metal grid in new clean cages or left undisturbed in their home cages and the number of fecal pellets excreted over a 60-min period was counted. Fecal pellet output was also measured in mice receiving an i.p. injection of neostigmine (2.5 μg/kg, i.p.), a reversible acetylcholinesterase inhibitor. Pharmacological treatments [2] GSK1016790A (0.001–0.1 mg/kg) or vehicle was injected intraperitoneally (i.p.) 15–75 min prior to the start of the assay. In selected experiments, RN 1734 was injected i.p. 15 min before agonist administration. Nitric oxide imaging [2] Nitric oxide (NO) imaging was performed as described (Supplementary Information). GSK1016790A (10−7 M) was bath applied for 30 s after a 5-min baseline period. Inhibitors were bath applied for the duration of the experiment and included RuR (10−6 M), SMTC (10−5 M), and 1400W (10−5 M). Additionally, the extracellular NO scavenger 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO, 10−4 M) was utilized. |
| 参考文献 |
|
| 其他信息 |
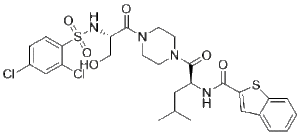
GSK1016790A is a tertiary carboxamide that is piperazine in which one of the amino groups has undergone condensation with the carboxy group of N-[(2,4-dichlorophenyl)sulfonyl]-L-serine, while the other has undergone condensation with the carboxy group of N-(1-benzothiophen-2-ylcarbonyl)-L-leucine. It is a cell-permeable, potent and selective agonist of the TRPV4 (transient receptor potential vanilloid 4) channel. It has a role as a TRPV4 agonist. It is a member of 1-benzothiophenes, a N-acylpiperazine, a sulfonamide, a dichlorobenzene, a tertiary carboxamide and an aromatic primary alcohol.
Identification of the TRPV4 Activator GSK1016790A. [[1] As part of a small molecule screening effort, GSK1016790A was identified as a novel TRPV4 channel activator (Fig. 1A). GSK1016790A potently induced Ca2+ influx in HEK cells expressing mouse TRPV4 channels with an EC50 value of 18 nM (Supplemental Fig. 2). GSK1016790A demonstrated a similar potency at human TRPV4 channels (EC50 = 2.1 nM). GSK1016790A was inactive against TRPV1 channels (see accompanying article, Willette et al., 2008), which, based on sequence homology, is the TRP superfamily.. GSK1016790A is a novel TRPV4 channel activator that is ∼300-fold more potent than the commonly used TRPV4 activator 4α-PDD. We have used molecular techniques, TRPV4−/− mice, in vitro contractility, and in vivo urodynamics in conjunction with GSK1016790A to support a role for TRPV4 in UBSM. TRPV4 mRNA was amplified by PCR, and protein expression was demonstrated by Western blot analysis and immunohistochemistry in UBSM and urothelium. GSK1016790A contracted bladder independent of the urothelium.. In the present study, a TRPV4-selective agonist GSK1016790A served as a tool to evaluate the role of TRPV4 in GI motility. Our goal was to characterize the effect of GSK1016790A on smooth muscle contractility and relaxation in the mouse intestine in vitro and in animal models of GI transit. In order to elucidate the intracellular pathways implicated in TRPV4-dependent signaling, calcium- and NO-imaging techniques were used. In addition, studies of tissues from healthy human subjects and patients with motility disorders were performed. Our data indicate that TRPV4 could become a viable target in the treatment of motility-related disorders in humans. [2] |
| 分子式 |
C28H32CL2N4O6S2
|
|---|---|
| 分子量 |
655.61
|
| 精确质量 |
654.114
|
| 元素分析 |
C, 51.30; H, 4.92; Cl, 10.81; N, 8.55; O, 14.64; S, 9.78
|
| CAS号 |
942206-85-1
|
| PubChem CID |
23630211
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
5.285
|
| tPSA |
176.23
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
10
|
| 重原子数目 |
42
|
| 分子复杂度/Complexity |
1070
|
| 定义原子立体中心数目 |
2
|
| SMILES |
CC(C)C[C@@H](C(=O)N1CCN(CC1)C(=O)[C@H](CO)NS(=O)(=O)C2=C(C=C(C=C2)Cl)Cl)NC(=O)C3=CC4=CC=CC=C4S3
|
| InChi Key |
IVYQPSHHYIAUFO-VXKWHMMOSA-N
|
| InChi Code |
InChI=1S/C28H32Cl2N4O6S2/c1-17(2)13-21(31-26(36)24-14-18-5-3-4-6-23(18)41-24)27(37)33-9-11-34(12-10-33)28(38)22(16-35)32-42(39,40)25-8-7-19(29)15-20(25)30/h3-8,14-15,17,21-22,32,35H,9-13,16H2,1-2H3,(H,31,36)/t21-,22-/m0/s1
|
| 化学名 |
N-[(2S)-1-[4-[(2S)-2-[(2,4-dichlorophenyl)sulfonylamino]-3-hydroxypropanoyl]piperazin-1-yl]-4-methyl-1-oxopentan-2-yl]-1-benzothiophene-2-carboxamide
|
| 别名 |
GSK1016790A; GSK-1016790A; GSK1016790A; 942206-85-1; GSK-1016790A; N-[(2S)-1-[4-[(2S)-2-[(2,4-dichlorophenyl)sulfonylamino]-3-hydroxypropanoyl]piperazin-1-yl]-4-methyl-1-oxopentan-2-yl]-1-benzothiophene-2-carboxamide; N-((S)-1-(4-((S)-2-(2,4-dichlorophenylsulfonaMido)-3-hydroxypropanoyl)piperazin-1-yl)-4-Methyl-1-oxopentan-2-yl)benzo[b]thiophene-2-carboxamide; CHEMBL4461515; (N-((1S)-1-{[4-((2S)-2-{[(2,4-Dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide; GSK 1016790A.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 33 mg/mL (~50.33 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (3.81 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (3.81 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (3.17 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5253 mL | 7.6265 mL | 15.2530 mL | |
| 5 mM | 0.3051 mL | 1.5253 mL | 3.0506 mL | |
| 10 mM | 0.1525 mL | 0.7626 mL | 1.5253 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。