| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
体外活性:肼屈嗪会损害 RAG-2 基因表达的上调并减少继发性 Ig 基因重排。肼屈嗪会破坏 B 淋巴细胞对自身的耐受性,并通过破坏受体编辑来促进致病性自身反应的产生。肼屈嗪直接清除游离丙烯醛,降低细胞内丙烯醛的可用性,从而抑制大分子加合。如果在开始接触丙烯醛后 30 分钟添加肼屈嗪,则会抑制交联,但如果在延迟 90 分钟后添加则无效。 Hydralazine (0.1-10 mM) 通过可能影响黄嘌呤氧化酶 (XO) 和烟酰胺腺嘌呤二核苷酸产生超氧自由基 (O(2)(*-)) 的 ROS 清除机制,抑制炎症巨噬细胞在细胞外和细胞内产生 ROS /烟酰胺腺嘌呤二核苷酸磷酸(NADH/NADPH)氧化酶。 Hydralazine (0.1-10 mM) 显着减少 NO(*) 的产生,这种效果可归因于抑制 NOS-2 基因表达和蛋白质合成。肼屈嗪还可以有效阻断 COX-2 基因表达,这与蛋白质水平和 PGE(2) 合成的降低完全相关。
|
||
|---|---|---|---|
| 体内研究 (In Vivo) |
肼屈嗪对小鼠血浆标记酶的增加提供强大的、剂量依赖性的保护,但不能防止烯丙醇引起的肝脏谷胱甘肽的消耗。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Taking oral hydralazine with food improves the bioavailability of the drug. An intravenous dose of 0.3mg/kg leads to an AUC of 17.5-29.4µM\*min and a 1mg/kg oral dose leads to an AUC of 4.0-30.4µM\*min. The Cmax of oral hydralazine is 0.12-1.31µM depending on the acetylator status of patients. <10% of hydralazine is recovered in the feces; 65-90% is recovered in the urine. The volume of distribution is 1.34±0.79L/kg in congestive heart failure patients and 1.98±0.22L/kg in hypertensive patients. The majority of hydralazine clearance is extrahepatic- 55% for rapid acetylators and 70% for slow acetylators. The average clearance in congestive heart failure patients is 1.77±0.48L/kg/h, while hypertensive patients have an average clearance of 42.7±8.9mL/min/kg. Metabolism / Metabolites Acetylation is a minor metabolic pathway for hydralazine; the major pathway is hydroxylation followed by glucuronidation. There are 5 identified metabolic pathways for hydralazine. Hydralazine can be metabolized to phthalazine or α-ketoglutarate hydrazone. These metabolites can be further converted to phthalazinone or hydralazine can be metabolized directly to phthalazinone. Hydralazine can undergo a reversible converstion to the active hydralazine acetone hydrazone. Hydralazine is spontaneously converted to the active pyruvic acid hydrazone or the pyruvic acid hydrazone tricyclic dehydration product, and these metabolites can convert back and forth between these 2 forms. Hydralazine can be converted to hydrazinophthalazinone, which is further converted to the active acetylhydrazinophthalazinone. The final metabolic process hydralazine can undergo is the conversion to an unnamed hydralazine metabolite, which is further metabolized to 3-methyl-s-triazolophthalazine (MTP). MTP can be metabolized to 9-hydroxy-methyltriazolophthalazine or 3-hydroxy-methyltriazolophthalazine; the latter is converted to triazolophthalazine. Hydralazine has known human metabolites that include hydralazine N-acetyl. Biological Half-Life Hydralazine has a half life of 2.2-7.8h in rapid acetylators and 2.0-5.8h in slow acetylators. The half life in heart failure patients is 57-241 minutes with an average of 105 minutes and in hypertensive patients is 200 minutes for rapid acetylators and 297 minutes for slow acetylators. Hydralazine is subject to polymorphic acetylation; slow acetylators generally have higher plasma levels of hydralazine and require lower doses to maintain control of pressure. However, other factors, such as acetylation being a minor metabolic pathway for hydralazine, will contribute to differences in elimination rates. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Serum aminotransferase elevations during hydralazine therapy are considered uncommon. However, hydralazine has been clearly linked to cases of acute liver injury with jaundice as well as a delayed lupus-like syndrome. Two clinical patterns of hepatic injury have been described, associated with either a short (2 to 6 weeks) or long (2 months to more than a year) latency period. The clinically apparent liver injury is usually hepatocellular, although cholestatic forms have also been reported (Case 1). In cases with a short latency period, rash, fever and eosinophilia are common and the onset is typically abrupt and severe, and recovery is rapid. In cases with a longer latency (Case 2), the onset is more typically insidious, liver biopsy may resemble chronic hepatitis and demonstrate fibrosis, and autoantibodies are often present. The late form of hepatitis may also accompany the lupus-like syndrome that occurs with hydralazine, particularly in high doses when given for 6 months or more. Recovery can be prolonged. Autoantibodies to isoforms of the P450 system (CYP 1A2) have been identified in patients with hepatotoxicity due to the structurally related antihypertensive agent dihydralazine (available in Europe, but not the United States) and which is associated with a higher rate of hepatotoxicity than hydralazine. Likelihood score: A (well established cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Limited milk level and infant serum level data and a long history of use in postpartum mothers indicate that hydralazine is an acceptable antihypertensive in nursing mothers, even those nursing newborns. ◉ Effects in Breastfed Infants No adverse effects reported in one infant breastfed for 8 weeks. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Hydralazine is 87% protein bound in serum likely to human serum albumin. |
||
| 参考文献 |
Proc Natl Acad Sci U S A.2007 Apr 10;104(15):6317-22;J Pharmacol Exp Ther.2004 Sep;310(3):1003-10.
|
||
| 其他信息 |
Hydralazine is the 1-hydrazino derivative of phthalazine; a direct-acting vasodilator that is used as an antihypertensive agent. It has a role as an antihypertensive agent and a vasodilator agent. It is a member of phthalazines, an azaarene, an ortho-fused heteroarene and a member of hydrazines.
Originally developed in the 1950s as a malaria treatment, hydralazine showed antihypertensive ability and was soon repurposed. Hydralazine is a hydrazine derivative vasodilator used alone or as adjunct therapy in the treatment of hypertension and only as adjunct therapy in the treatment of heart failure. Hydralazine is no longer a first line therapy for these indications since the development of newer antihypertensive medications. Hydralazine hydrochloride was FDA approved on 15 January 1953. Hydralazine is an Arteriolar Vasodilator. The physiologic effect of hydralazine is by means of Arteriolar Vasodilation. Hydralazine is a commonly used oral antihypertensive agent that acts by inducing peripheral vasodilation. Hydralazine has been linked to several forms of acute liver injury as well as a lupus-like syndrome. Hydralazine has been reported in Achillea pseudopectinata with data available. Hydralazine is a phthalazine derivative with antihypertensive effects. Hydralazine exerts its vasodilatory effects through modification of the contractile state of arterial vascular smooth muscle by altering intracellular calcium release, and interfering with smooth muscle cell calcium influx. This agent also causes inhibition of phosphorylation of myosin protein or chelation of trace metals required for smooth muscle contraction, thereby resulting in an increase in heart rate, stroke volume and cardiac output. A direct-acting vasodilator that is used as an antihypertensive agent. See also: Hydralazine Hydrochloride (has salt form). Drug Indication Hydralazine is indicated alone or adjunct to standard therapy to treat essential hypertension. A combination product with isosorbide dinitrate is indicated as an adjunct therapy in the treatment of heart failure. Mechanism of Action Hydralazine may interfere with calcium transport in vascular smooth muscle by an unknown mechanism to relax arteriolar smooth muscle and lower blood pressure. The interference with calcium transport may be by preventing influx of calcium into cells, preventing calcium release from intracellular compartments, directly acting on actin and myosin, or a combination of these actions. This decrease in vascular resistance leads to increased heart rate, stroke volume, and cardiac output. Hydralazine also competes with protocollagen prolyl hydroxylase (CPH) for free iron. This competition inhibits CPH mediated hydroxylation of HIF-1α, preventing the degradation of HIF-1α. Induction of HIF-1α and VEGF promote proliferation of endothelial cells and angiogenesis. |
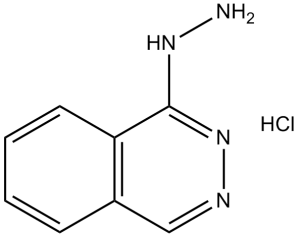
| 分子式 |
C8H8N4.HCL
|
|
|---|---|---|
| 分子量 |
196.63686
|
|
| 精确质量 |
196.051
|
|
| CAS号 |
304-20-1
|
|
| 相关CAS号 |
Hydralazine;86-54-4
|
|
| PubChem CID |
3637
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 沸点 |
491.9ºC at 760 mmHg
|
|
| 熔点 |
273°C
|
|
| 闪点 |
251.3ºC
|
|
| LogP |
1.724
|
|
| tPSA |
63.1
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
1
|
|
| 重原子数目 |
12
|
|
| 分子复杂度/Complexity |
150
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
0
|
|
| InChi Key |
ZUXNZUWOTSUBMN-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C8H8N4.ClH/c9-11-8-7-4-2-1-3-6(7)5-10-12-8;/h1-5H,9H2,(H,11,12);1H
|
|
| 化学名 |
phthalazin-1-ylhydrazine; hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (10.58 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (10.58 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: 8.33 mg/mL (42.36 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶 (<60°C). 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.0854 mL | 25.4272 mL | 50.8544 mL | |
| 5 mM | 1.0171 mL | 5.0854 mL | 10.1709 mL | |
| 10 mM | 0.5085 mL | 2.5427 mL | 5.0854 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03514108 | Recruiting | Drug: Hydralazine Isosorbide Dinitrate Drug: Metformin Hydrochloride |
Heart Failure Diabetes |
Henrik Wiggers | March 1, 2018 | Phase 4 |
| NCT00607477 | Terminated Has Results | Drug: Minoxidil Drug: Hydralazine |
Treatment Induced Hypertension | University of Chicago | January 2008 | Not Applicable |
| NCT02522208 | Completed | Drug: BiDil XR Drug: BiDil Immediate Release (IR) |
Heart Failure | Arbor Pharmaceuticals, Inc. | September 2015 | Phase 1 |
| NCT02933593 | Withdrawn | Drug: Labetalol Drug: Hydralazine |
Hypertension | St. Louis University | August 2016 | Not Applicable |
|
|
|