| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
大戟的活性成分巨大戟二萜醇甲丁酸酯(也称为巨大戟醇 3-当归酯)是一种强效 PKC 激活剂,PKC-α、PKC-β、PKC 的 Kis 值为 0.3、0.105、0.162、0.376 和 0.171 nM -γ、PKC-δ 和 PKC-ε,按此顺序。 Ingenium 二甲酚甲基丁酯的 EC50 为 13 ± 2.4 nM (PKC-α)、4.37 ± 0.4 nM (PKC-βI)、10.5 ± 2.2 nM (PKC-βII)、38.6 ± 2.9 nM (PKC-δ)、1.08 ± 0.01 nM (PKC-ε)、0.9 ± 0.13 nM (PKC-μ)、198 ± 12.5 nM (PKC-α)、69.1 ± 8.2 nM (PKC-βI)、WEHI-231 细胞中 4.6 ± 0.4 nM (PKC) -ε) 和 1 nM (PKC-μ)、635 ± 245 nM (PKC-α)、146 ± 35 nM (PKC-βI)、4.7 ± 0.7 nM (PKC-δ)、HOP-92 细胞中 1.1 ± 0.5 Colo-205 细胞中为 nM (PKC-ε) 和 30 nM (PKC-μ)。 WEHI-231 细胞、HOP-92 和 Colo-205 细胞被巨大戟二萜醇甲丁酸酯致敏,IC50 值依次为 1.41 ± 0.255 nM、3.24 ± 2.01 nM 和 11.9 ± 1.307 nM [1]。 PKC-δ 依赖性巨大戟二萜醇甲丁酯 (PEP005;20 nM) 可导致原发性 AML 骨髓母细胞凋亡,但不会导致正常成髓细胞凋亡 [2]。巨大戟二萜醇甲丁酯 (PEP005) 会抑制 PKCδ,而激活 PKCα。与亲本 Colo205-S 细胞相比,Colo205-R 细胞的 IC50 > 10 μM,这使得它们对巨大戟二萜醇甲基丁酯的耐药性高出 300 倍以上 [3]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Since ingenol mebutate is a topical treatment, the systemic absorption is less than 0.1 ng/mL. There is no route of elimination since ingenol mebutate is a topical treatment. There is no volume of distribution quantity since ingenol mebutate is a topical treatment. There is no clearance quantity since ingenol mebutate is a topical treatment. Plasma clearance and volume of distribution (steady-state) in humans were estimated using a simple allometric correlation based on body weight. Using a one-compartment model with first-order absorption and elimination kinetics, it was estimated that dermal administration of the maximum intended clinical dose of 2 ug/kg/day would produce levels of ingenol mebutate in the blood below the LLOQ of 0.1 ng/mL. Blood clearance and volume of distribution at steady-state were predicted to range from approximately 0.22 to 1.01 L/hr/kg and approximately 0.61 L/kg, respectively. The absorption rate constant and topical bioavailability was projected to be 0.0277 hours-1 and 0.21%, respectively. A human blood Tmax of 2 hours and Cmax of 0.107 pg/mL were predicted for a 2 ug/kg/day topical dose. A minimum topical dose of 2000 ug/kg/day to humans would be required produce detectable blood levels. After IV administration, a high to very high blood clearance, moderate to high volume of distribution at steady-state and short half-life were observed in rats, rabbits, dogs and minipigs. Following IV administration of 3(H)-ingenol mebutate to rats, drug-related radioactivity was well distributed to the tissues and there were no gender differences in the organs exposed but elimination was faster in females. In vitro, ingenol mebutate and its isomers were shown to have high plasma protein binding in rats, dogs, minipigs and humans (>99%). In rats, the majority of an intravenous dose of 3(H)-ingenol mebutate was excreted via the biliary route, with urinary excretion as a minor pathway. After in vitro applications of 0.01%, 0.1% or 0.05% PEP005 Gels to rat, mini-pig and human skin preparations, the percutaneous absorption was generally low, with a range of 0.04% (mini-pig) to 8.68% (rat) across animal species and 0.16% to 1.93% in humans. The absorbed doses of 3(H)-ingenol mebutate were in the order of WI rat > SD rat > human > mini-pig. After topical administration of PEP005 Gel to mini-pigs, blood levels of ingenol mebutate were generally not detected, and when detected, ranged up to 0.1 ng/mL. After topical administration of ingenol mebutate to rats, blood levels were consistently quantifiable only at doses of 300 ug/kg or greater, in which case the absolute bioavailability was 2% to 4%. The systemic exposure to Picato gel, 0.05% was assessed in two studies in a total of 16 subjects with AK, following application of approximately 1 g of Picato gel, 0.05% to an area of 100 cm2 of the dorsal forearm once daily for two consecutive days. In these studies, the blood levels of ingenol mebutate and two of its metabolites (acyl isomers of ingenol mebutate) were measured. Blood levels of ingenol mebutate and the two metabolites were below the lower limit of quantification (0.1 ng/mL) in all the blood samples of the subjects evaluated. Metabolism / Metabolites There is no metabolism of Picato since ingenol mebutate is a topical treatment, and ingenol mebutate does not inhibit or induce a majority of the cytochrome P450 (CYP) enzymes. The in vitro metabolism of ingenol mebutate was qualitatively similar in blood, skin homogenates and hepatocytes of rats, dogs, minipigs and humans. Ingenol mebutate was found to be relatively stable in blood and skin homogenates, and to undergo extensive metabolism in cryopreserved hepatocytes. The major pathway in rat, dog and minipig hepatocytes was hydrolysis to ingenol, whereas the major pathway in humans was hydroxylation of ingenol mebutate. In the skin of rats, dogs, minipigs and humans, rearrangement of ingenol mebutate was predominantly to PEP015 (approximately 26% to approximately 31%) and, to a much lesser extent, PEP025 (approximately 1% to approximately 2%); hydrolysis to ingenol was minimal (0% to 0.81%). However, after topical or IV administration of ingenol mebutate to rats and minipigs, PEP025 was not detected and PEP015 was less than 10% of the corresponding ingenol mebutate concentration in the blood. Biological Half-Life There is no half-life quantity since ingenol mebutate is a topical treatment. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Ingenol mebutate is a white to pale yellow crystalline powder. As the drug Picato, it is used as a gel for the topical treatment of actinic keratosis. HUMAN EXPOSURE AND TOXICITY: Results from three pharmacology studies in healthy volunteers indicate a favorable topical safety profile for ingenol mebutate gel, with no evidence seen of skin sensitization, photoirradiation, or photoallergic potential. However, topical overdosing could result in an increased incidence of local skin reactions. ANIMAL STUDIES: In rats given repeat IV dosing for 28 days, treatment-related effects included transient tachypnea, which was not dose-related, lethargy and/or subdued behavior and decreased food consumption. In mice dosed for 7 consecutive days, one animal receiving 60 ug/kg/day and all animals receiving >/= 80 ug/kg/day were killed prematurely after Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Topical ingenol mebutate has not been studied during breastfeeding. However, after topical administration, serum concentrations were undetectable, so breastfeeding is not expected to result in exposure of the breastfed infant. If ingenol mebutate is required by the mother, it is not a reason to discontinue breastfeeding. Do not apply ingenol mebutate to the breast or nipple and ensure that the infant's skin does not come into direct contact with the areas of skin that have been treated. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding There is no plasma protein binding quantity since ingenol mebutate is a topical treatment |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Ingenol mebutate is included in the database. Picato gel is indicated for the topical treatment of actinic keratosis. /Included in US product label/ EXPL Although anti-retroviral therapy (ART) is highly effective in suppressing HIV replication, it fails to eradicate the virus from HIV-infected individuals. Stable latent HIV reservoirs are rapidly established early after HIV infection. Therefore, effective strategies for eradication of the HIV reservoirs are urgently needed. We report that ingenol-3-angelate (PEP005), the only active component in a previously FDA approved drug (Picato) for the topical treatment of precancerous actinic keratosis, can effectively reactivate latent HIV in vitro and ex vivo with relatively low cellular toxicity. Biochemical analysis showed that PEP005 reactivated latent HIV through the induction of the pS643/S676-PKCdelta-8-1kappaBalpha/epsilon-NF-kappaB signaling pathway. Importantly, PEP005 alone was sufficient to induce expression of fully elongated and processed HIV RNAs in primary CD4+ T cells from HIV infected individuals receiving suppressive ART. Furthermore, PEP005 and the P-TEFb agonist, JQ1, exhibited synergism in reactivation of latent HIV with a combined effect that is 7.5-fold higher than the effect of PEP005 alone. Conversely, PEP005 suppressed HIV infection of primary CD4+ T cells through down-modulation of cell surface expression of HIV co-receptors. This anti-cancer compound is a potential candidate for advancing HIV eradication strategies. EXPL /The purpose of this study was/ to evaluate the safety of two applications of PEP005 (ingenol mebutate) gel in superficial basal cell carcinoma. Efficacy was a secondary end-point. Randomized, vehicle-controlled, phase IIa study conducted at eight private dermatology clinics in Australia /evaluated/ a total of 60 patients with histologically confirmed superficial basal cell carcinoma (lesion size, 4-15 mm). /They/ were randomized to treatment on days 1 and 2 (Arm A) or days 1 and 8 (Arm B) and, within each arm, to ingenol mebutate gel, 0.0025%, 0.01% or 0.05%, or vehicle gel. The main outcome measures were the incidence and severity of adverse events and local skin responses in Arms A and B; lesion clearance at day 85 was a secondary measure. The incidence of adverse events was low. One patient treated with ingenol mebutate gel, 0.05% in Arm A experienced severe flaking/scaling/dryness extending beyond the application site. Non-severe, potentially treatment-related events included erythema extending beyond the application site, application-site pain and headache in two patients each. Six patients in Arm A had one or more severe local skin responses. Efficacy appeared to be dose-related and there was a trend towards higher clinical and histological lesion clearance rates in Arm A compared with Arm B. Histological clearance occurred in five of eight patients (63%) randomized to ingenol mebutate gel, 0.05% in Arm A. Two applications of ingenol mebutate gel, 0.05%, are safe and have efficacy in patients with superficial basal cell carcinoma. Drug Warnings Hypersensitivity reactions, including anaphylaxis and allergic contact dermatitis, have been reported post-marketing. If anaphylactic or other clinically significant hypersensitivity reactions occur, discontinue Picato gel immediately and institute appropriate medical therapy. Avoid treatment in the periocular area. Eye disorders, including severe eye pain, chemical conjunctivitis, corneal burn, eyelid edema, eyelid ptosis, periorbital edema can occur after exposure. The following adverse reactions have been identified during post approval use of Picato (ingenol mebutate) gel, 0.015% and 0.05%: hypersensitivity, allergic contact dermatitis, herpes zoster, chemical conjunctivitis, and corneal burn. Severe skin reactions in the treated area, including erythema, crusting, swelling, vesiculation/postulation, and erosion/ulceration, can occur after topical application of Picato gel. Administration of Picato gel is not recommended until the skin is healed from any previous drug or surgical treatment. For more Drug Warnings (Complete) data for Ingenol mebutate (12 total), please visit the HSDB record page. Pharmacodynamics The pharmacodynamics of ingenol mebutate in producing cell death in actinic keratosis is unknown. |
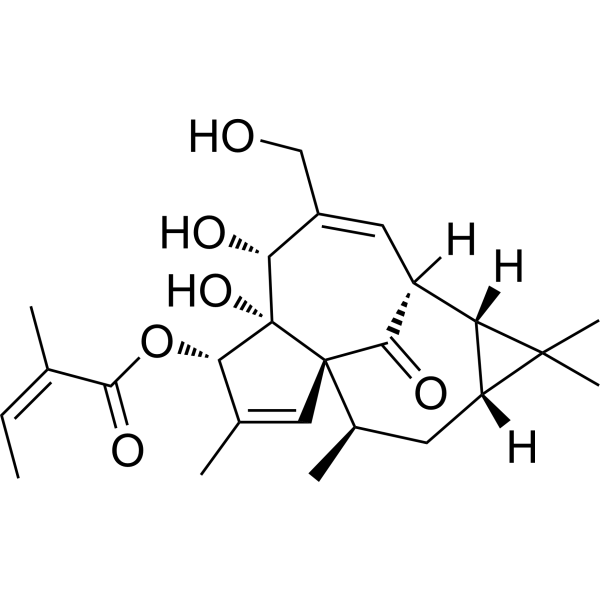
| 分子式 |
C25H34O6
|
|---|---|
| 分子量 |
430.53
|
| 精确质量 |
430.235
|
| CAS号 |
75567-37-2
|
| 相关CAS号 |
Ingenol;30220-46-3
|
| PubChem CID |
6918670
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
576.9±50.0 °C at 760 mmHg
|
| 闪点 |
191.4±23.6 °C
|
| 蒸汽压 |
0.0±3.6 mmHg at 25°C
|
| 折射率 |
1.594
|
| LogP |
5.18
|
| tPSA |
104.06
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
31
|
| 分子复杂度/Complexity |
926
|
| 定义原子立体中心数目 |
8
|
| SMILES |
C/C=C(/C)\C(=O)O[C@H]1C(=C[C@@]23[C@@]1([C@@H](C(=C[C@H](C2=O)[C@H]4[C@H](C4(C)C)C[C@H]3C)CO)O)O)C
|
| InChi Key |
VDJHFHXMUKFKET-WDUFCVPESA-N
|
| InChi Code |
InChI=1S/C25H34O6/c1-7-12(2)22(29)31-21-13(3)10-24-14(4)8-17-18(23(17,5)6)16(20(24)28)9-15(11-26)19(27)25(21,24)30/h7,9-10,14,16-19,21,26-27,30H,8,11H2,1-6H3/b12-7-/t14-,16+,17-,18+,19-,21+,24+,25+/m1/s1
|
| 化学名 |
[(1S,4S,5S,6R,9S,10R,12R,14R)-5,6-dihydroxy-7-(hydroxymethyl)-3,11,11,14-tetramethyl-15-oxo-4-tetracyclo[7.5.1.01,5.010,12]pentadeca-2,7-dienyl] (Z)-2-methylbut-2-enoate
|
| 别名 |
PEP 005; PEP-005; PEP005
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 100 mg/mL (~232.27 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.81 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.81 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.81 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3227 mL | 11.6136 mL | 23.2272 mL | |
| 5 mM | 0.4645 mL | 2.3227 mL | 4.6454 mL | |
| 10 mM | 0.2323 mL | 1.1614 mL | 2.3227 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Ingenol Mebutate Gel, 0.015% Repeat Use for Multiple Actinic Keratoses on Face and Scalp
CTID: null
Phase: Phase 3 Status: Completed
Date: 2012-04-26