| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Adenosine A2A receptor ( Ki = 2.2 nM )
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:伊曲茶碱对 A2AR 的亲和力比对 A1 受体的亲和力高 70 倍,Ki 分别为 2.2 nM 和 150 NM。原代大鼠纹状体星形胶质细胞暴露于 Istradefylline 会导致体外 bFGF 诱导星形胶质细胞增生的浓度依赖性消除。伊曲茶碱对人中 A1 受体、A2A 受体和 A3 受体的结合亲和力 (Ki) 分别为 >287 nM、9.12 nM 和 > 681 nM,对大鼠中 A1 受体和 A2A 受体分别为 50.9 nM 和 1.57 nM,小鼠中 A1 受体和 A2A 受体分别为 105.02 nM 和 1.87 nM。细胞测定:将永久表达人腺苷 A1 或 A2A 受体的 CHO 细胞系培养在补充有 10% (v/v) 胎牛血清、50 U/mL 青霉素和 50 μg/mL 链霉素的 α-MEM 中。细胞在 37°C、5% CO2 的环境中生长。将这些细胞以 15,000 个细胞/孔的密度接种在黑色 96 孔测定板上,然后培养 24 小时。
|
| 体内研究 (In Vivo) |
Istradefylline 可逆转 CGS21680 诱导的和利血平诱导的僵直症,ED50 分别为 0.05 mg/kg 和 0.26 mg/kg。与其他腺苷拮抗剂和多巴胺激动剂药物相比,伊曲茶碱在这些模型中的效力超过 10 倍。伊曲茶碱与左旋多巴 (50 mg/kg) 联合用药对氟哌啶醇诱发和利血平诱发的僵直症具有显着效果。对 MPTP 处理的普通狨猴口服 10 mg/kg Istradefylline 可使运动活动增加至对照的约两倍,并改善运动残疾。伊曲茶碱(10 mg/kg,口服,SKF80723/喹吡罗/L-多巴前 90 分钟)与 SKF80723(1 mg/kg,腹膜内注射)、喹吡罗(0.06 mg/kg 腹膜内注射)或 L-DOPA(2.5 mg/kg po)对运动活动和运动残疾的改善产生显着的累加效应,但对运动障碍没有影响。在 MPTP 小鼠模型中,Istradefylline 在各种条件下都能显着减弱纹状体多巴胺的消耗。在单剂量 MPTP 之前用 Istradefylline (3.3 mg/kg, ip) 进行预处理可减弱 1 周后在纹状体中测量到的部分多巴胺和 DOPAC 消耗。口服 Istradefylline 可防止大鼠中 6-羟基多巴胺诱导的黑质多巴胺能神经元细胞丧失,并防止小鼠纹状体中多巴胺能神经末梢的功能丧失以及随后由 MPTP 引起的神经胶质增生。长期伊曲茶碱治疗不能改善多巴胺耗尽大鼠的逆转缺陷。联合服用伊曲茶碱或托吡卡胺可显着减少匹莫齐特引起的下颌颤抖。匹莫齐特诱导的腹外侧纹状体 c-Fos 表达增加可被行为有效剂量的伊曲茶碱降低,与托吡卡胺相反,匹莫齐特治疗的大鼠中 c-Fos 表达实际上增加。
|
| 细胞实验 |
人腺苷 A1 或 A2A 受体在 CHO 细胞系中永久表达,该细胞系在补充有 10% (v/v) 胎牛血清、50 U/mL 青霉素和 50 μg/mL 链霉素的 α-MEM 中培养。在 5% CO2 环境中,细胞在 37°C 下生长。这些细胞以每孔 15,000 个细胞的密度接种在黑色 96 孔测定板上后培养 24 小时。
|
| 动物实验 |
The animals are kept in standard housing with a 12-hour light-dark cycle, at a temperature of 24-26°C and a relative humidity of 50–60%. They can be kept in pairs or alone. The diet was composed of fresh fruit, standard food pellets, and marmoset jelly from Mazuri. For five days, the animals receive a daily dosage of 2.0 mg/kg sc of MPTP. The animals are given six to eight weeks to recuperate from the acute effects of MPTP treatment. The animals are hand-fed Mazuri marmoset jelly and fresh fruit puree during MPTP treatment and in the ensuing weeks until they are able to sustain themselves. Prior to behavioral testing, all animals exhibit a significant decrease in basal locomotor activity, slower and less coordinated movements, abnormal postures of some body parts, and a reduction in blinking and checking movements between 6-8 weeks and 8 months after exposure to MPTP. By oral gavage, istradefylline (KW-6002) is given in a final volume of 2.0 mL/kg body weight after being suspended in a solution of 0.3% Tween-80 and 10% sucrose.
Researchers evaluated the efficacy and potency of istradefylline (KW-6002) and other reference compounds in the selective adenosine A2A receptor agonist 2-[p-(2-carboxyethyl)phenethylamino]-5'-N-ethylcarboxamidoadenosin e (CGS 21680)-, haloperidol- or reserpine-induced catalepsy models. The effect of KW-6002 on reserpine or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride(MPTP)-induced hypolocomotion was also examined. Results: The ED50s of KW-6002 in the reversal of CGS21680-induced and reserpine-induced catalepsy were 0.05 mg/kg, PO and 0.26 mg/kg, PO, respectively. Compared to the ED50 of other adenosine antagonists and dopamine agonist drugs, KW-6002 is over 10 times as potent in these models. istradefylline (KW-6002) also ameliorated the hypolocomotion (minimum effective dose; 0.16 mg/kg) induced by nigral dopaminergic dysfunction with MPTP or reserpine treatment. Combined administrations of subthreshold doses of KW-6002 and L-dopa (50 mg/kg, PO) exerted prominent effects on haloperidol-induced and reserpine-induced catalepsy, suggesting that there may be a synergism between the adenosine A2A receptor antagonist KW-6002 and dopaminergic agents.[2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Istradefylline reaches a Cmax of 181.1ng/mL with a Tmax of 2.0h and an AUC of 11,100ng\*h/mL. M1, the primary active metabolite, reaches a Cmax of 4.34ng/mL with a Tmax of 3.5h. The M8 metabolite reaches a Cmax of 12.6ng/mL with a Tmax of 3.0h and an AUC of 610ng\*h/mL. A 3mg/kg oral dose given to male rats was 17.6% elminated in the urine and 68.3% eliminated in the feces. In urine, 5.31% of the total dose was the M3 metabolite and 1.96% of the total dose was the M1 metabolite. In feces, 30.60% of the total dose was the M3 metabolite, 9.34% of the total dose was the M1 metabolite, 8.33% of the total dose was the M10 metabolite, and 1.62% of the total dose was unchanged istradefylline. The apparent volume of distribution of istradefylline is 448-557L. The apparent clearance of istradefylline is 4.1-6.0L/h. Metabolism / Metabolites The primary metabolite found in urine is the active 4'-O-monodesmethyl istradefylline (M1). Istradefylline is metabolized mainly by CYP1A1, CYP3A4, and CYP3A5. CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C18, and CYP2D6 also partly contribute the the metabolism of istradefylline. Other identified metabolites are 1-β-hydroxylated-4’-O-demethyl istradefylline (M2), 3’,4’-O-didemethyl istradefylline (M3), M1 sulfate conjugate (M4), M1 glucuronide (M5), 1-β-hydroxylated istradefylline (M8) and hydrogenated M3 (M10). Biological Half-Life The terminal elimination half life of istradefylline was 64-69 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In prelicensure controlled trials, serum ALT elevations occurred in 4% to 11% of istradefylline-treated subjects compared to 5% to 6% of placebo recipients, most of whom were taking multiple other agents for Parkinson disease. The elevations were usually mild-to-moderate in severity, asymptomatic and self-limited in course. ALT elevations above 3 times the ULN occurred in less than 1% recipients and rarely led to discontinuation. None of the aminotransferase elevations were accompanied by symptoms or jaundice. In preregistration clinical trials and subsequently with its more widespread use, istradefylline has not been linked to instances of clinically apparent liver injury. It has, however, had limited clinical use. Likelihood score: E (unlikely cause of clinically apparent liver injury). Protein Binding Istradefylline is approximately 98% protein bound in plasma, mostly to serum albumin and alpha-1-acid glycoprotein. |
| 参考文献 | |
| 其他信息 |
Pharmacodynamics
Istradefylline is a selective adenosine A2A receptor inhibitor. It has a long duration of action as it is given once daily and has a half life of 64-69 hours. Patients taking this medication should be monitored for dyskinesia, hallucinations, and lack of impulse control. Consider dose reductions for these patients. |
| 分子式 |
C20H24N4O4
|
|
|---|---|---|
| 分子量 |
384.43
|
|
| 精确质量 |
384.179
|
|
| 元素分析 |
C, 62.49; H, 6.29; N, 14.57; O, 16.65
|
|
| CAS号 |
155270-99-8
|
|
| 相关CAS号 |
Istradefylline-13C,d3; 2749234-46-4
|
|
| PubChem CID |
5311037
|
|
| 外观&性状 |
Light green to green solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
601.0±65.0 °C at 760 mmHg
|
|
| 熔点 |
189-193
|
|
| 闪点 |
317.3±34.3 °C
|
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
|
| 折射率 |
1.598
|
|
| LogP |
2.84
|
|
| tPSA |
80.28
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
6
|
|
| 重原子数目 |
28
|
|
| 分子复杂度/Complexity |
613
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
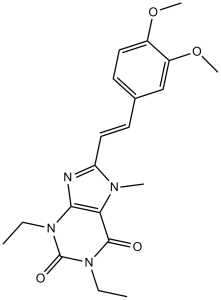
O=C1C2=C(N=C(/C=C/C3=CC(OC)=C(OC)C=C3)N2C)N(CC)C(N1CC)=O
|
|
| InChi Key |
IQVRBWUUXZMOPW-PKNBQFBNSA-N
|
|
| InChi Code |
InChI=1S/C20H24N4O4/c1-6-23-18-17(19(25)24(7-2)20(23)26)22(3)16(21-18)11-9-13-8-10-14(27-4)15(12-13)28-5/h8-12H,6-7H2,1-5H3/b11-9+
|
|
| 化学名 |
8-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-1,3-diethyl-7-methylpurine-2,6-dione
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.41 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 配方 2 中的溶解度: 30% Propylene glycol , 5% Tween 80 , 65% D5W: 30mg/mL 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6013 mL | 13.0063 mL | 26.0125 mL | |
| 5 mM | 0.5203 mL | 2.6013 mL | 5.2025 mL | |
| 10 mM | 0.2601 mL | 1.3006 mL | 2.6013 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05333549 | Recruiting | Drug: Istradefylline | Parkinson Disease Cognitive Impairment |
Virginia Commonwealth University | July 18, 2022 | Phase 2 |
| NCT05182151 | Active Recruiting |
Drug: Istradefylline 40 mg | Parkinson Disease Apathy |
Medical University of South Carolina |
April 6, 2021 | N/A |
| NCT05217498 | Not yet recruiting | Drug: Istradefylline Device: low oxygen therapy |
Spinal Cord Injuries Myelopathy |
Randy Trumbower, PT, PhD | September 1, 2024 | Phase 1 Phase 2 |
| NCT05377424 | Recruiting | Drug: Consume 20mg of istradefylline Other: Low Oxygen therapy |
ALS | University of Florida | June 21, 2022 | Phase 1 Phase 2 |